
Concept explainers
(a)
Interpretation:
The principal product formed when
Concept introduction:
The general mechanism of electrophilic addition is as follows:
Step1:
Step2: Then the nucleophilic part of reagent adds to the carbocation to give the addition product.
These steps can be illustrated as follows:
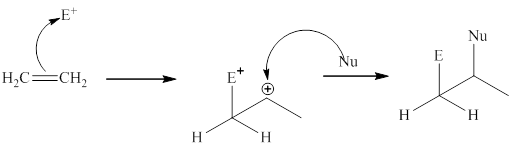
(b)
Interpretation:
The principal product formed in the monochlorination of propane should be predicted and written.
Concept introduction:
The reaction between chlorine and propane under ultraviolet light is the monohalogenation reaction. This is essentially a substitution reaction. Every possible monochlorinated products are formed. The major one is the one where chlorine is attached at the terminal carbon.
The principal product in the halogenation of
(c)
Interpretation:
The principal product when isopropyl alcohol is heated with benzoic acid should be predicted and written.
Concept introduction:
Heating alcohol and acid involve esterification reaction and an ester is formed along with the release of a water molecule.
The reaction can be illustrated as:

Where
The general mechanistic pathway for esterification as proposed by Fischer is as follows:
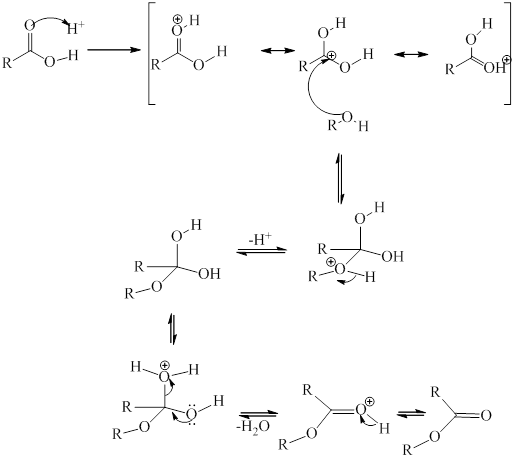
(d)
Interpretation:
The principal product when
Concept introduction:
Oxidation of alcohols can be done with an oxidizing reagent.
Tertiary alcohols cannot be oxidized as they do not have hydrogen; the secondary alcohols are oxidized to
While the primary alcohols oxidized to
The general formula of a tertiary, secondary and primary alcohol is represented as follows:
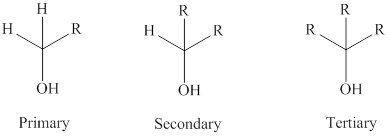
Want to see the full answer?
Check out a sample textbook solution
Chapter 27 Solutions
General Chemistry: Principles and Modern Applications (11th Edition)
- Write structural formulas for compounds that meet the following descriptions:(a) An alkene, C6H12, that cannot have cis–trans isomersand whose longest chain is 5 carbons long(b) An alkene with a chemical formula of C10H12 that hascis–trans isomers and contains a benzene ring.arrow_forward(1) Write a complete chemical equation showing reactants, products, and catalysts needed (if any) for the following reaction and (2) Draw and name the organic compound found in every reaction. (a) Complete hydrogenation of 2-Methylhexa-1,5-diene (b) Complete halogenation (Br2) of 3-Ethyl-2,2-dimethylhept-3-ene (c) Reaction of (4E)-2.4-Dimethylhexa-1,4-diene with a mole of water (d) Reaction of cis-3,3-Dimethyl-4-propylocta-1,5-diene with two mole of HBr (e) Reaction of trans-1-Bromo-3-chlorocyclopentane with potassium hydroxide (f) Formation of Gilman reagent using isopropyl bromide (g) Ozonolysis of 3,3-Dimethyloct-4-yne (h) Complete halogenation (Cl2) of 3-Ethyl-5-methyl-1,6,8-decatriyne (i) Partial hydrogenation using Lindlar's Catalyst 2,2,5,5-Tetramethylhex-3-yne (i) Reaction of 3.4-Dimethylcyclodecyne with sodium amidearrow_forward4. Write the formula and name for the product when cyclopentene reacts with (a) Cl2(b) HBr(c) H2, Pt (d) H2O, H+arrow_forward
- Give one example for the hydration of Alkenes: that is: the addition of H2O by Hydroboration.arrow_forward(a) When a compound containing C, H, and O is completelycombusted in air, what reactant besides the hydrocarbonis involved in the reaction? (b) What products form in thisreaction? (c) What is the sum of the coefficients in the balancedchemical equation for the combustion of one mole ofacetone, C3H6O1l2, in air?arrow_forward(a) What structural feature is associated with each type of hy-drocarbon: an alkane; a cycloalkane; an alkene; an alkyne? (b) Give the general formula for each type .(c) Which hydrocarbons are considered saturated?arrow_forward
- Reaction of butane (CH 3CH 2CH 2CH 3) with Cl 2 in the presence of light forms two different alkyl chlorides that have molecular formula C 4H 9Cl. Draw the structures of both alkyl chlorides.arrow_forwardArrange the following compounds in increasing order of their property as indicated :(i) CH3COCH3, C6H5COCH3, CH3CHO(reactivity towards nucleophilic addition reaction)(ii) Cl—CH2—COOH, F—CH2—COOH, CH3—COOH (acidic character)arrow_forward34) The product formed by the reaction of toluene with chlorine in the presence of sunlight is: (a) o-chlorotoluene (b) 2,4-dichlorotoluene (c) p-chlorotoluene (d) Benzylchloridearrow_forward
- Draw the organic product formed when the following compounds undergo a substitution reaction: (a) acetic acid and 1-hexanol; (b) propanoic acid and dimethyl-amine; (c) ethanoic acid and diethylamine.arrow_forward7. The general formula for noncyclic alkenes is: (a) CH-2 (b) CH (c) CH2 (d) CH (e) CH,arrow_forwardTRUE OR FALSE (a) Both ethylene and acetylene are planar molecules. (b) An alkene in which each carbon of the double bond has two different groups bonded to it will show cis-trans isomerism. (c) Cis-trans isomers have the same molecular formula but a different connectivity of their atoms. (d) Cis-2-butene and trans -2-butene can be interconverted by rotation about the carbon–carbon double bond. (e) Cis-trans isomerism is possible only among appropriately substituted alkenes. (f) Both 2-hexene and 3-hexene can exist as pairs of cis-trans isomers. (g) Cyclohexene can exist as a pair of cis-trans isomers. (h) 1-Chloropropene can exist as a pair of cis-trans isomers.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





