
Concept explainers
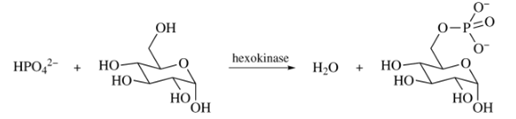
The phosphorylation of
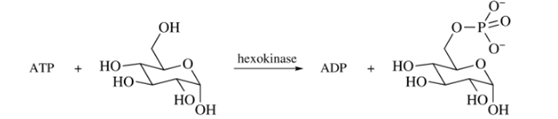
Is this reaction exergonic or endergonic?
How would the value of
Use the value for the hydrolysis of ATP to ADP (Section 27.5) to calculate
Want to see the full answer?
Check out a sample textbook solution
Chapter 27 Solutions
Organic Chemistry - Standalone book
- For a given acid HA, it was determined that at pH 6.0 the concentration of the conjugate base [A] was 0.075 M and the acid [HA] was 0.025 M. What percent of this acid is ionized at pH 6.0? What is the pKa of this acid? What pH would this acid be 50% lonized?arrow_forwardDetermine the direction that each of the reactions will progress. Assume that the reactants and products are present in equimolar amounts. The standard free energy of hydrolysis of ATP is –30.5 kJ/mol. fructose+ATP ____fructose 6‑phosphate+ADP The standard free energy of hydrolysis for fructose 6‑phosphate is −15.9 kJ/mol. 3‑phosphoglycerate+ATP___1,3‑bisphosphoglycerate+ADP The standard free energy of hydrolysis for 1,3‑bisphosphoglycerate is −49.3 kJ/mol. creatine+ATP___creatine phosphate+ADP The standard free energy of hydrolysis for creatine phosphate is –43.0 kJ/mol.arrow_forwardThe number of ATP molecules consumed and produced during glycolysis under anaerobic conditions are summarized in the following diagram (the ATP hydrolysis and synthesis reactions are shown in green). Phosphate Carbon atom 2 ATP 2 ADP ●●●●●● Glucose I Energy-Investing Phase O 2 ATP O 6 ATP O 1 ATP 8 ATP NADH + NAD* +H* O 4 ATP ●●●● 2 ADP 2 ATP NADH + NAD +H Based on the diagram above, for each glucose molecule broken down, what is the net energy yield of glycolysis in terms of ATP under anaerobic conditions? Pyruvate 2 ADP 2 ATP ●●●●● Energy-Generating Phase Pyruvatearrow_forward
- Consider the malate dehydrogenase reaction from the citric acid cycle. Given the listed concentrations, calculate the free energy change for this reaction at energy change for this reaction at 37.0 °C (310 K). AG' for the reaction is +29.7 kJ/mol. Assume that the reaction occurs at pH 7. [malate] = 1.25 mM [oxaloacetate] = 0.130 mM [NAD+] = 440 mM [NADH] = = 180 mM kJ.mol-¹ AG: X10 TOOLSarrow_forwardIntracellular concentrations in resting muscle are as follows: fructose- 6-phosphate, 1.0 mM; fructose-1,6-bisphosphate, 10 mM; AMP, 0.1 mM; ADP, 0.5 mM; ATP, 5 mM; and Pi, 10 mM. Is the phosphofructokinase reaction in muscle more or less exergonic than under standard conditions? By how much?arrow_forwardWhen the following reaction reached equilibrium the concentration of glucose 1-phosphate is 34mM, and the concentration of glucose 6-phosphate is 190mM. At standard temperatures and pressure, calculate the Keq and the ΔGo' glucose 6-phosphate <---(phosphoglucoisomerase)---> glucose 1-phosphate Keq= ΔGo' =arrow_forward
- If Glutamate + NH4 --> glutamine Delta G = 14.2 kj/mol and ATP + H2O --> ADP + Pi Delta G = -30.5 kj/mol What is true? - overall reaction is non-spontaneous -reaction 2 is non-spontaneous - overall reaction is spontaneous -reaction 1 is spontaneous.arrow_forwardConsider a uniport system where a carrier protein transports an uncharged substance A across a cell membrane. Suppose that at a certain ratio of [A]inside to [Aloutside , the AG for the transport of substance A from outside the cell to the inside, Aoutside - Ajnside is 14.9 KJ/mol at 25°C. What is the ratio of the concentration of substance A inside the cell to the concentration outside? [Alinside [AJoutsidearrow_forwardChemistry Calculate the standard free-energy for creatine-phosphate hydrolysis in a cell where: creatine-P = 60mmol, creatine = 30 mmol, ATP = 1 mmol, ADP = 0.1 mmol. Is this reaction exer/endergonic?arrow_forward
- A solution of the enzyme glycogen synthase incubated at 45°C lost 70% of its activity in 3 minutes „but when incubated at 45°C in the presence of a very large concentration of its substrate UDP- Glucose, it lost only 3% of its activity in the same amount of time, Why? Clu CH,OH CH, OH HO, он CH;OH CH2OH но HO он HO UDP O-P-0 OH OH NH o--o CH, OH CH2OH CH,OH OH OH HO UDP-glucose binding makes it easier for the enzyme to unfold. The enzyme likely had phosphate groups added through covalent modification. O Interactions with the bound UDP-glucose stabilize the tertiary structure of glycogen synthase. The UDP-Glucose is a positíve effector which increases the enzyme activity.arrow_forwardAnswer True or False The Na/K-ATPase pump is an example of a coupling element between a spontaneous process and a non spontaneous process.arrow_forwardThe formation of glutamine from glutamate and ammonium ions requires 14.2 kJ/mol of energy input. The reaction is driven by energy from hydrolysis of ATP to ADP (A,G = -31). mol How many mols of ATP must be hydrolyzed to produce enough energy to make 1 mol of glutamine?arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning


