
Concept explainers
Oligonucleotide Synthesis
In Section 27.6 we noted that synthetic oligonucleotides of defined sequence were commercially
available for use as primers for PCR and as probes for cloning DNA. Here we will examine how these oligonucleotides are prepared.
The method bears many similarities to the Merrifield solid-phase synthesis of peptides. A starter unit is attached to a solid support, nucleosides are attached one-by-one until the sequence is complete, whereupon the target oligonucleotide is removed from the support and purified. Like solid-phase peptide synthesis, the preparation of oligonucleotides relies heavily on protecting groups and bond-forming methods.
The starter units are nucleosides in which
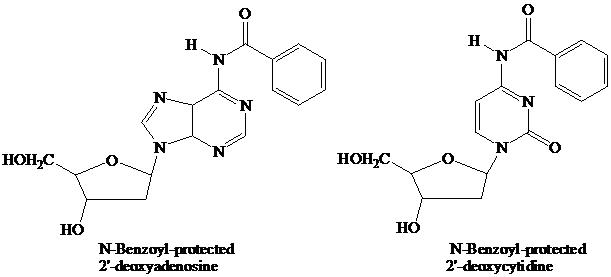
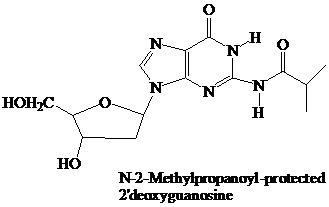
Thymidine lacks an −NH2 group, so needs no protecting group on its pyrimidine base.
These N-protecting groups remain in place throughout the synthesis. They are the first ones added and the last ones removed. None of the further “chemistry” that takes place involves the purine or pyrimidine rings.
The 5'-OH group of the 2'-deoxyribose portion of the nucleosides is primary and more reactive toward ether formation than the 3'-OH group, which is secondary. This difference allows selective protection of the 5'-OH as its 4,4'-dimethoxytriphenylmethyl
(DMT) ether.
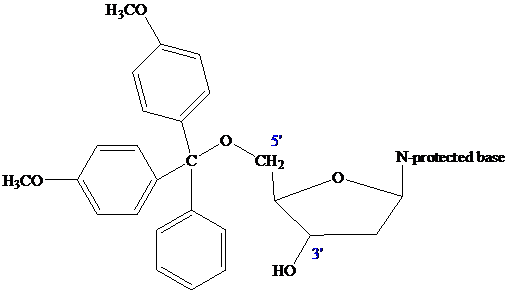
The nucleoside that is to serve as the 3' end of the final oligonucleotide is attached to a
controlled-pore glass (CPG) bead by ester formation between its unprotected 3'-OH and a linker unit already attached to the CPG. In order for chain elongation to proceed in the 3'→5' direction, the DMT group that protects the 5'-OH of the starter unit is removed by treatment with dichloroacetic acid.

The stage is now set for adding the second nucleoside. The four blocked nucleosides prepared
earlier are converted to their corresponding 3'-phosphoramidite
derivatives. An appropriate A, C, T, or G phosphoramidite is used in each successive stage of the elongation cycle.
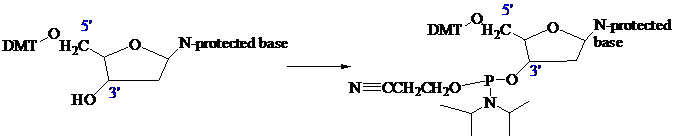
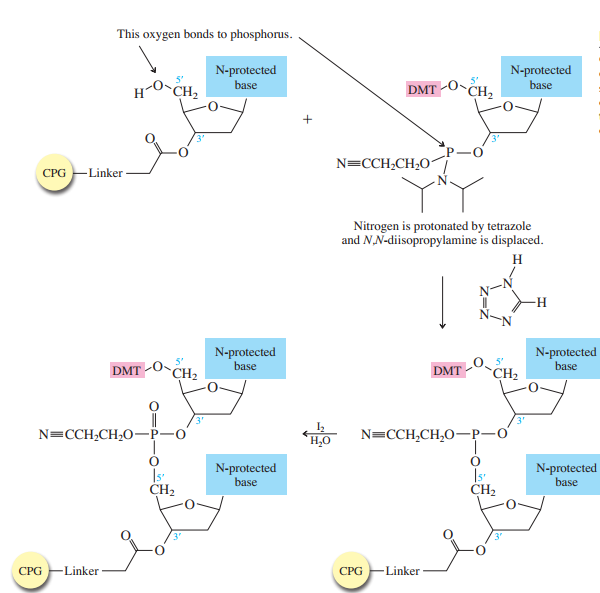
Each phosphoramidite is coupled to the anchored nucleoside by a reaction in which the free 5'-OH of the anchored nucleoside displaces the diisopropylamino group from phosphorus (Figure 27.15).The coupling is catalyzed by tetrazole, which acts as a weak acid to protonate the diisopropylamino group.
The product of the coupling is a phosphite; it has the general formula P(OR)3. It is oxidized to phosphate [P(O)(OR)3]
in the last step of Figure 27.15.
The 5'-OH of the newly added nucleoside is then deprotected to prepare the bound dinucleotide for the next elongation cycle.

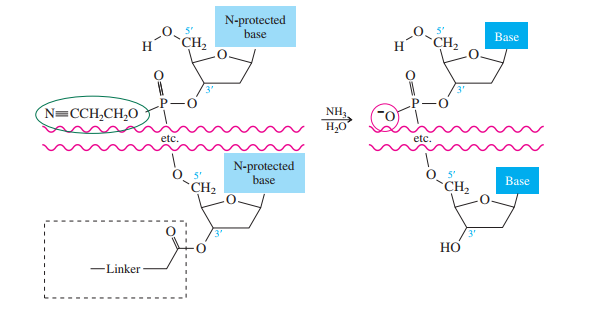
Once all the nucleosides are in place and the last DMT is removed, treatment with aqueous
ammonia removes the acyl and cyanoethyl groups and cleaves the oligonucleotide from the CPG
support.
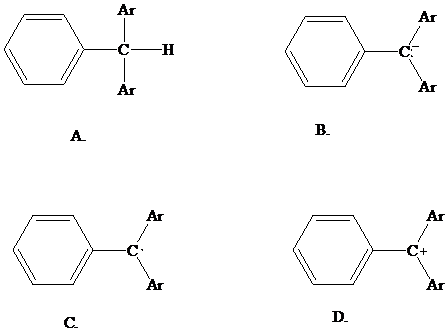
27.30
What species is formed from the DMT -
protecting group when it is removed using dichloroacetic acid? (Ar = p-CH3OC6H4)
Section 27.6
Many important compounds contain two or more nucleotides joined together by
a phosphodiester linkage. The best known are those in which the phosphodiester
joins the
5′-oxygen of one
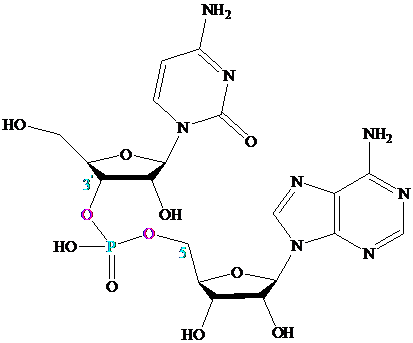
Oligonucleotides contain about 50 or fewer nucleotides held together by
phosphodiester links; polynucleotides can contain thousands of nucleotides.
Want to see the full answer?
Check out a sample textbook solution
Chapter 27 Solutions
Organic Chemistry - Standalone book
- Nonearrow_forwardConsider the structure of 1-bromo-2-fluoroethane. Part 1 of 2 Draw the Newman projection for the anti conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond. ✡ ぬ Part 2 of 2 H H F Br H H ☑ Draw the Newman projection for the gauche conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond. H F Br H Harrow_forwardPlease help me answer this question. I don't understand how or where the different reagents will attach and it's mostly due to the wedge bond because I haven't seen a problem like this before. Please provide a detailed explanation and a drawing showing how it can happen and what the final product will look like.arrow_forward
- Which of the following compounds is the most acidic in the gas phase? Group of answer choices H2O SiH4 HBr H2Sarrow_forwardWhich of the following is the most acidic transition metal cation? Group of answer choices Fe3+ Sc3+ Mn4+ Zn2+arrow_forwardBased on the thermodynamics of acetic acid dissociation discussed in Lecture 2-5, what can you conclude about the standard enthalpy change (ΔHo) of acid dissociation for HCl? Group of answer choices You cannot arrive at any of the other three conclusions It is a positive value It is more negative than −0.4 kJ/mol It equals −0.4 kJ/molarrow_forward
- Add conditions above and below the arrow that turn the reactant below into the product below in a single transformation. + More... If you need to write reagents above and below the arrow that have complex hydrocarbon groups in them, there is a set of standard abbreviations you can use. More... T H,N NC Datarrow_forwardIndicate the order of basicity of primary, secondary and tertiary amines.arrow_forward> Classify each of the following molecules as aromatic, antiaromatic, or nonaromatic. Cl Z- N O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic O nonaromatic O aromatic ○ antiaromatic nonaromaticarrow_forward

 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





