
Concept explainers
Interpretation:
The given compounds caffeine and theobromine are to be classified as pyrimidine and purine. The fact that one of these two cannot isomerize to an enolic form, while two different enols are possible for the other is to be explained. The structural formulas for the possible enols is to be written.
Concept introduction:
The purines and pyrimidines are nitrogen bases that constitute the structural unit of
Both purines and pyrimidines are nitrogen containing heterocyclic
The structures of pyrimidine and purine are shown below:
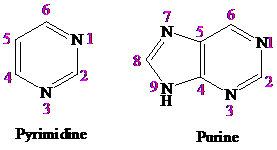
The structure of pyrimidine resembles that of benzene and pyridine having two nitrogen atoms.
In the structure of purine, the prymidine ring is fused with imidazole ring and has four nitrogen atoms.
The structure having an acidic hydrogen at alpha position to
Want to see the full answer?
Check out a sample textbook solution
Chapter 27 Solutions
Organic Chemistry - Standalone book
- 1. Complete each of the following by supplying the missing reagents. Draw thestructures of each of the reactants and products.a. N-methylpropanamide + ? → methanamine + ?b. Formamide + strong acid → ? + ? 2. Write the structure of the following: N-Propylhexanamide Propionamide N-Methylpropanamidearrow_forwardComplete the synthesis of the following compounds by writing the reagent/reaction condition, or by drawing the structure of the missing organic substrate/product.arrow_forward1. What chemical structural differences are there between the monoterpenes and alkaloids? 2. What do you understand by ‘biogenic isoprene rule’? Who proposed these rule and what is the significance of these rule?arrow_forward
- SYNTHESIS OF ESTERS VIA NUCLEOPHILIC ACYL SUBSTITUTION Write the chemical equation involved in the reaction between the excess acid and NaHCO3. Explain why NaHCO3 is preferred over NaOH for the neutralization of excess acid. How was excess alcohol eliminated from the crude product.arrow_forwardBile salts are derivative of cholestrol. However, the solubilities of these compounds in water are drastically different; cholestrol is highly hydrophobic, and the bile salts are soluble in digestive juices. Explain the differences.arrow_forwardAnilines can be converted into diazonium salts by reaction with nitrous acid. Diazonium salts react with phenols to form azo dyes. Sketch the aniline derivative and phenol reactant that can combine to produce the azo dye shown.arrow_forward
- The substance provided can be prepared by a nucleophilic addition reaction between an aldehydeor ketone and nucleophile. Identify the reactants from which it was prepared. If the substance is an acetal, identify the carbonyl compound and the alcohol; if it is an imine or enamine, identify the carbonyl compound and the amine.arrow_forwardDescribe the reaction process of the synthesis of Dilantin from benzil.arrow_forwardExplain amine catalysis in urethane bond formationarrow_forward
- Describe the components required for the synthesis of this dihydropyridine, MeO. EtO₂C. ZI CO₂Mearrow_forwardFeatures of the structure of imino acids. Formation of amides from amino acids.arrow_forwardImidazole boils at 257 °C, whereas N-methylimidazole boils at 199 °C. Explain the difference in boiling points.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning



