
Concept explainers
Oligonucleotide Synthesis
In Section
available for use as primers for PCR and as probes for cloning DNA. Here we will examine how these oligonucleotides are prepared.
The method bears many similarities to the Merrifield solid-phase synthesis of peptides. A starter unit is attached to a solid support, nucleosides are attached one-by-one until the sequence is complete, whereupon the target oligonucleotide is removed from the support and purified. Like solid-phase peptide synthesis, the preparation of oligonucleotides relies heavily on protecting groups and bond-forming methods.
The starter units are nucleosides in which
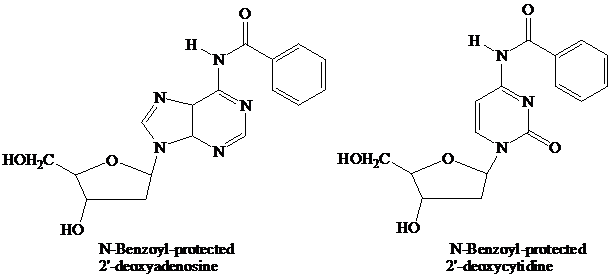
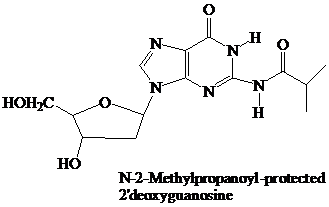
Thymidine lacks an
These
remain in place throughout the synthesis. They are the first ones added and the last ones removed. None of the further “chemistry” that takes place involves the purine or pyrimidine rings.
The
(DMT) ether.
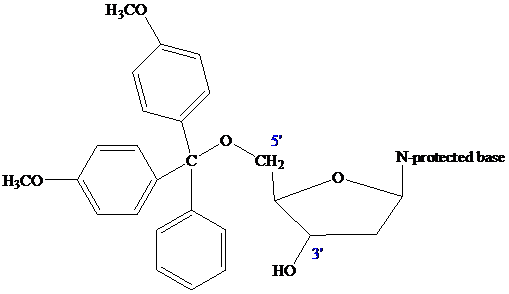
The nucleoside that is to serve as the
controlled-pore glass (CPG) bead by ester formation between its unprotected

The stage is now set for adding the second nucleoside. The four blocked nucleosides prepared
earlier are converted to their corresponding
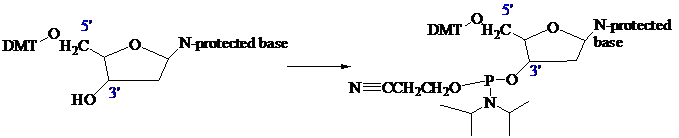
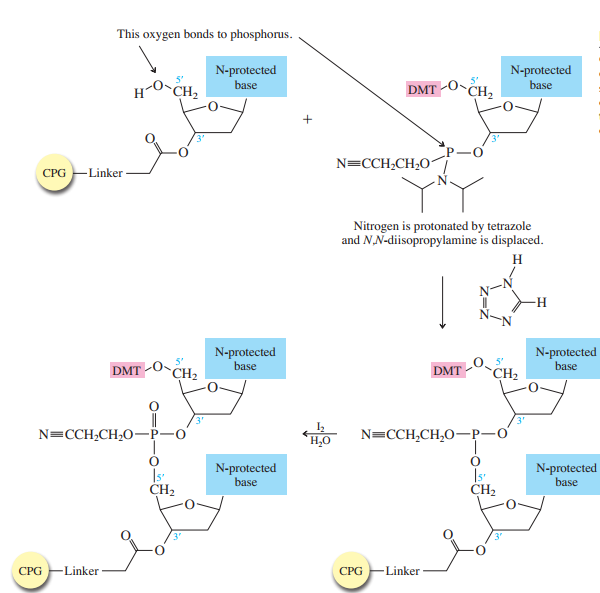
Each phosphoramidite is coupled to the anchored nucleoside by a reaction in which the free
The product of the coupling is a phosphite; it has the general formula
in the last step of Figure
The

Once all the nucleosides are in place and the last DMT is removed, treatment with aqueous
ammonia removes the acyl and cyanoethyl groups and cleaves the oligonucleotide from the CPG
support.
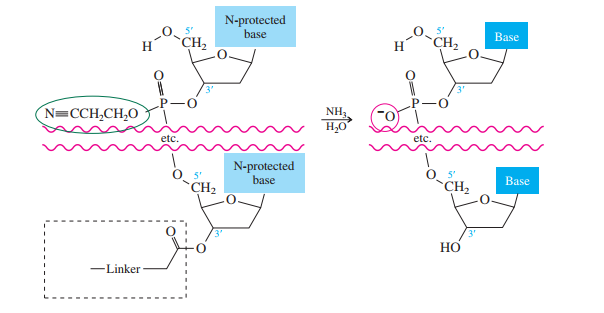
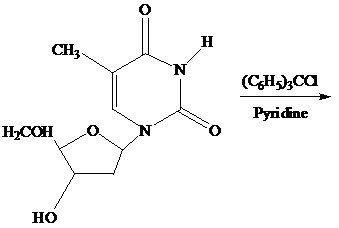
What is the product of the following reaction?
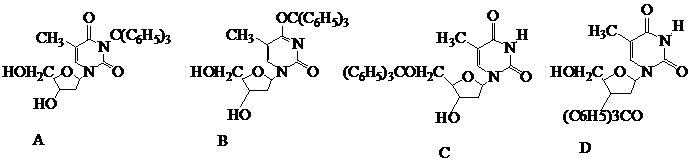
Section
Many important compounds contain two or more nucleotides joined together by
a phosphodiester linkage. The best known are those in which the phosphodiester
joins the
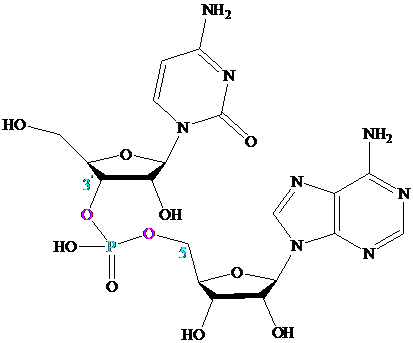
Oligonucleotides contain about
phosphodiester links; polynucleotides can contain thousands of nucleotides.
Want to see the full answer?
Check out a sample textbook solution
Chapter 27 Solutions
Organic Chemistry - Standalone book
- Determine the amino acid sequence of a polypeptide from the following data: Complete hydrolysis of the peptide yields Ala, Arg, Gly, 2 Lys, Met, Phe, Pro, 2 Ser, Tyr, and Val. Treatment with Edman’s reagent releases PTH-Val. Carboxypeptidase A releases Ala. Treatment with cyanogen bromide yields the following two peptides: 1. Ala, 2 Lys, Phe, Pro, Ser, Tyr 2. Arg, Gly, Met, Ser, Val Treatment with trypsin yields the following three peptides: 1. Gly, Lys, Met, Tyr 2. Ala, Lys, Phe, Pro, Ser 3. Arg, Ser, Val Treatment with chymotrypsin yields the following three peptides: 1. 2 Lys, Phe, Pro 2. Arg, Gly, Met, Ser, Tyr, Val 3. Ala, Serarrow_forwardThree peptides were obtained from a trypsin digestion of two different polypeptides. indicate the possible sequences from the given data and tell what further experiment should be carried out in order to determine the primary structure of the polypeptide. polypeptide I: 1. Val-Gly-Asp-Lys 2. Leu-Glu-Pro-Ala-Arg 3. Ala-Leu-Gly-Asparrow_forwardShow how solid-phase peptide synthesis would be used to make Ile-gly-asnarrow_forward
- 1. How does real-time polymerase chain reaction work? 2. What does the melting point represent?arrow_forwardDetermine the amino acid sequence of a polypeptide from the following data: Acid-catalyzed hydrolysis gives Ala, Arg, His, 2 Lys, Leu, 2 Met, Pro, 2 Ser, Thr, and Val. Carboxypeptidase A releases Val. Edman’s reagent releases PTH-Leu. Treatment with cyanogen bromide gives three peptides with the following amino acid compositions: 1. His, Lys, Met, Pro, Ser 2. Thr, Val 3. Ala, Arg, Leu, Lys, Met, Ser Trypsin-catalyzed hydrolysis gives three peptides and a single amino acid: 1. Arg, Leu, Ser 2. Met, Pro, Ser, Thr, Val 3. Lys 4. Ala, His, Lys, Metarrow_forwardThe following peptide is incubated with Trypsin. Draw the resultant fragments at pH = 5. Be sure to include all atoms. Peptide: LRFAKEarrow_forward
- Deduce the sequence of a pentapeptide that contains the amino acids Ala, Glu, Gly, Ser, and Tyr, from the following experimental data. Edman degradation cleaves Gly from the pentapeptide, and carboxypeptidase forms Ala and a tetrapeptide. Treatment of the pentapeptide with chymotrypsin forms a dipeptide and a tripeptide. Partial hydrolysis forms Gly, Ser, and the tripeptide Tyr–Glu–Ala.arrow_forward3) Indicate the product of the following stages of peptide synthesis by drawing the appropriate molecule in the box provided. polymer bead CF CO2H HO. N=C=N (DCC)arrow_forwardGiven: Alanine: pKa=4 Pkb=9 Histidine: pKa=2 pKb=6 and pKc=9 Taking the structure of the amino acids into account, give a comparative discussion of these pK values.arrow_forward
- Predict the fragmentation pattern and masses of the dipeptide productarrow_forwardDenaturation of a protein is a physical change, the most readily observable result of which is loss of biological activity. Denaturation stems from changes in secondary, tertiary, and quaternary structure through disruption of noncovalent interactions including hydrogen bonding and hydrophobic interactions. Three common denaturing agents are sodium dodecyl sulfate (SDS), urea, and heat. What kinds of noncovalent interactions might each reagent disrupt?arrow_forwardHydrolysis of decapeptide P with the enzyme trypsin affords the following fragments: Gly-Asp, Ala-Cys-Lys, Leu-Trp- Val-Gly-Arg. Hydrolysis with chymotrypsin yields: Leu-Trp, Val-Gly-Arg-Ala-Cys-Lys-Gly-Asp. What is the amino acid sequence of P?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





