
Concept explainers
(a)
Interpretation:
The name of following molecule with appropriate stereochemical designation should be determined:
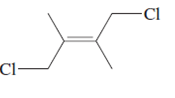
Concept introduction:
The E-configuration stands for anti-configuration, whereas, Z-configuration stands for same side configuration.
The determination of configuration is done on the basis of the
(b)
Interpretation:
The name of following molecule with appropriate stereochemical designation should be determined:
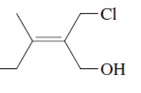
Concept introduction:
Alkenes are unsaturated hydrocarbons with double covalent bond between carbon-carbon atoms. On the basis of groups bonded with the double bonded carbon atoms, alkenes can be classified as E and Z-configuration.
The E-configuration stands for anti-configuration, whereas, Z-configuration stands for same side configuration.
The determination of configuration is done on the basis of the atomic/molecular mass of the atoms/groups attached to double bonded carbon atoms. If both higher atomic/molecular mass atom/groups are placed at the same side, then it is said to be Z-configuration and in E-configuration, these groups will be at anti-position.
(c)
Interpretation:
The name of following molecule with appropriate stereochemical designation should be determined:
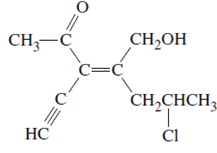
Concept introduction:
Alkenes are unsaturated hydrocarbons with double covalent bond between carbon-carbon atoms. On the basis of groups bonded with the double bonded carbon atoms, alkenes can be classified as E and Z-configuration.
The E-configuration stands for anti-configuration, whereas, Z-configuration stands for same side configuration.
The determination of configuration is done on the basis of the atomic/molecular mass of the atoms/groups attached to double bonded carbon atoms. If both higher atomic/molecular mass atom/groups are placed at the same side, then it is said to be Z-configuration and in E-configuration, these groups will be at anti-position.
(d)
Interpretation:
The name of following molecule with appropriate stereochemical designation should be determined:
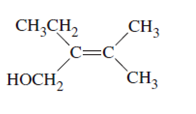
Concept introduction:
Alkenes are unsaturated hydrocarbons with double covalent bond between carbon-carbon atoms. On the basis of groups bonded with the double bonded carbon atoms, alkenes can be classified as E and Z-configuration.
The E-configuration stands for anti-configuration, whereas, Z-configuration stands for same side configuration.
The determination of configuration is done on the basis of the atomic/molecular mass of the atoms/groups attached to double bonded carbon atoms. If both higher atomic/molecular mass atom/groups are placed at the same side, then it is said to be Z-configuration and in E-configuration, these groups will be at anti-position.
Want to see the full answer?
Check out a sample textbook solution
Chapter 26 Solutions
General Chemistry: Principles and Modern Applications (11th Edition)
- Consider the molecule 1-bromo-2-methylbutane. C3 and C4 should be drawn as Et as in theexample. This group is called an ethyl group and can be considered a sphere about twice the sizeof a methyl group. Draw the following Newman projections sighting down the C1C2 bond... a. The lowest potential energy conformation. b. The highest potential energy staggered conformation.arrow_forwardInterconversion of the staggered and eclipsed conformations of alkanes requires rotation around a -C bond such as the one depicted below (see arrow). Using your knowledge of bonding, explain why these rotations do not significantly affect the energy (strength) of these bonds. You may find it helpful to describe the type of bond being rotated.arrow_forwardWhich of the following is the most stable conformation of bromocyclohexane? IV.arrow_forward
- Name the following molecule by following IUPAC rules. Use appropriate stereochemical designations (R, S, E, or Z) as needed. Do not put spaces between dashes or commas in the answer.arrow_forwardDraw all the possible conformations of trans- and cis-1,3-dimethylcyclobutane. Which of these conformations is the most stable conformation? Explain your answer. Discuss the type of strain(s) that affect the stability of these compounds.arrow_forwardWhat is the systematic name for the molecule depicted by the following bond line structure? Decide what the longest C-C chain is (parent) and then decide which substituents are there and how to number it to give the lowest locants. Make sure each substituent gets a locant. Separate letters from numbers by a dash, and numbers from each other by a comma. Do not put any spaces in your name.arrow_forward
- Determine which cyclohexane structure has the MOST energy (is the LEAST stable)?arrow_forwardDraw the line bond structures for the following alkenes, cyclic alkenes, and alkynes: Can you explain to me about this part A) noncyclic alkenes that contain 4 carbon atoms (3 possible), please? Can you explain to me about this part B) cyclic alkenes that contain 4 carbon atoms (4 possible), please? Can you explain to me about this part C) alkynes that contain 4 carbon atoms (2 possible, neither of them is a cyclic alkyne), please?arrow_forwardThe energy difference between a tert-butyl group going from equatorial to axial in a cyclohexane is 18.3 kJ/mol. When two of the carbon atoms are replaced with oxygen atoms (molecule B) the energy difference between the two chair conformations drops to 5.9 kJ/mol. Explain this difference. (Hint: Consider what makes putting groups axial unfavorable).arrow_forward
- How many isomers (structural, geometric, and stereo) have the formula C5H₁0, and have "cyclo" in their name? (i.e. they contain a ring) 6 4 7 3 5arrow_forwardball & stick v + labels Which of the Newman structures below represents this conformation of 3-chloro-2-methylpentane, as viewed along the C2-C3 bond? ***** CH3 Et H Et Et. .CI H3C. CH3 Et. H3C. H3C H. TCI H3C CH3 H. ČH3 H ČH3 a b d (Enter the letter(s) of the correct structure(s), in alphabetical order and without punctuation; more than one answer may be correct. Et = an ethyl group)arrow_forwardWrite TRUE if the BOLD word/phrase makes the statement correct. Otherwise, write the correct WORD/PHRASE that will make the statement true. If there are two bold words/phrases in a number, write your answer for EACH of the bold words/phrases. 1. The anti-staggered conformation of butane, in which the methyl groups have a dihedral angle of less than 180°, has the highest energy. 2. The formation of 2-methylpropene as side product in the synthesis of tert-butyl chloride is a nucleophilic substitution reaction. 3. The generation of tertiary carbocation is the rate-determining step in tert-butyl chloride synthesis.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
