
Life: The Science of Biology
11th Edition
ISBN: 9781319010164
Author: David E. Sadava, David M. Hillis, H. Craig Heller, Sally D. Hacker
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 2, Problem 4Q
Summary Introduction
To review:
The following structure of acetone and whether it affects the bicarbonate or carbonic acid buffer system in the blood.
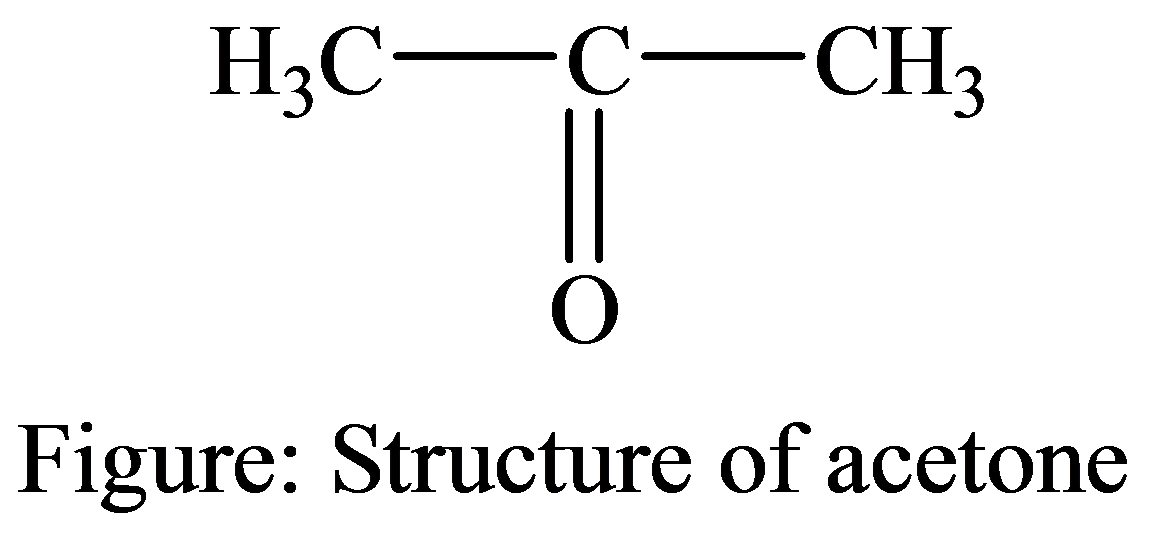
Introduction:
Acetone is also known as propanone, which is the organic compound, and its appearance is colorless. It comes in a form of volatile and inflammable liquid and it is known to be the simplest and tiniest
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Give full explanation
The physcian orders ascorbic acid 0.25mg IM for your patient admitted with an alcohol problem. You have ascorbic acid 500mg/mL. How many milliliters will you administer?
A patient is suspected of having low stomach acid, a condition known as hypochloridia. To determine whether the patient
has this condition, her doctors take a 18.00 mL sample of her gastric juices and titrate the sample with
4.85 × 10-4 M KOH. The gastric juice sample required 1.26 mL of the KOH titrant to neutralize it.
Calculate the pH of the gastric juice sample. Assume the sample contained no ingested food or drink which might
otherwise interfere with the titration.
pH =
For the patient to be suffering from hypochloridia, the pH of the gastric juices from the stomach must be greater than pH 4.
Does the patient have hypochloridia?
unable to determine
no
yes
A patient is suspected of having low stomach acid, a condition known as hypochloridia. To determine whether the patient has
this condition, her doctors take a 18.00 mL sample of her gastric juices and titrate the sample with 3.94 x 10-4 M KOH. The
gastric juice sample required 6.60 mL of the KOH titrant to neutralize it.
Calculate the pH of the gastric juice sample. Assume the sample contained no ingested food or drink which might otherwise
interfere with the titration.
pH =
Enter numeric value
For the patient to be suffering from hypochloridia, the pH of the gastric juices from the stomach must be greater than pH 4.
Does the patient have hypochloridia?
no
O unable to determine
O yes
Chapter 2 Solutions
Life: The Science of Biology
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- You receive a prescription for Epanutin® Suspension. The patient normally takes 150mg daily in capsule form, however he has recently started to have difficulty in swallowing them. The doctor asks you to check that the Epanutin® Capsules and Suspension are equivalent to each other. The following information is extracted from the BNF. Dose equivalence and conversion Preparations containing phenytoin sodium are not bioequivalent to those containing phenytoin base (such as Epanutin® suspension); 100 mg of phenytoin sodium is approximately equivalent in therapeutic effect to 92 mg phenytoin base. Epanutin 30mg/5ml oral suspension (Pfizer Ltd) Phenytoin 6 mg per 1 ml Phenytoin sodium 100mg capsules (Actavis UK Ltd) Phenytoin sodium 100 mg What daily dose of Epanutin® Suspension should be written on the dispensing label (in ml)? (Round to the nearest ml) units -mlarrow_forwardThe concentration ofacetylcholine (a neurotransmitter) in a sample can be determined from the pH changes thataccompany its hydrolysis. When the sample is incubated with the enzymeacetylcholinesterase, acetylcholine is converted to choline and acetic acid, whichdissociates to yield acetate and a hydrogen ion: In a typical analysis, 15 mL of an aqueous solution containing an unknown amount ofacetylcholine had a pH of 7.65. When incubated with acetylcholinesterase, the pH of thesolution decreased to 6.87. Assuming there was no buffer in the assay mixture, determinethe number of moles of acetylcholine in the 15 mL sample.arrow_forwardCalculate the pH of a blood plasma sample with a total CO₂ concentration of 25.7 mM and bicarbonate concentration of 24.4 mM. The relevant pK, of carbonic acid is 6.1. Enter the answer with three significant figures. pH =arrow_forward
- A pharmacist is asked to compound the following for the preparation of 100 capsules: Estriol 200 mg Estrone 25 mg Estradiol 25 mg Using a balance that has an SR of 6 mg, the aliquot method of weighing, lactose as the diluent, and an error in weighing of 4%, show, by calculations, how the correct quantity of estrone can be obtained to accurately compound the formula.arrow_forwardShown below is the titration curve for phosphoric acid. At what pH is the solution entirely in the H3PO4 form? 0-0.5 14 12.5 O2.1 NICHarrow_forwardAnswer the following problem in relation with Carboxylic Acids: The bones of a murdered 9-year-old boy were found lying on his father's property. Immediately the father was suspected of having killed the child. A sample of the soil under the boy's bones was sent to Dr. Vass for analysis. No volatile fatty acids were found in the soil sample. What can you conclude from this data?arrow_forward
- Discuss the suitability of bromocresol green and thymol blue (2nd change only) for use as indicators in an acetic acid - sodium hydroxide titration. Paragraph в I A + v ...arrow_forwardLipids from an organic sample are extracted separately by column chromatography using the ff eluants: 1st eluant - chloroform: methanol: water (6:3:1) 2nd eluant - methanol: chloroform (9:1) 3rd eluant – petroleum ether: ethyl ether (1:1) Test results for each eluate are tabulated below. Identify the possible lipid present in each eluate. Test Reagents 1. KHSO4 2. HNO3,(NH4)3MoO4 3. CHCl3, acetic anhydride, conc. H2SO4 4. -naphthol, conc. H2SO4 5. Hydroxylamine HCl, FeCl3 6. ninhydrin reagent POSSIBLE LIPIDS: Triacylglycerol, cholesterol, sphingomyelin, lecithinarrow_forwardA buffer contains 0.015 mol of lactic acid (pK₁ = 3.86) and 0.080 mol of sodium lactate per liter. H₂C OH Lactic acid OH Calculate the pH of the buffer. H₂C. Calculate the change in pH after adding 9.0 mL of 0.10 M HCl to 1.0 L of the buffer. O OH Sodium lactate Calculate the change in pH after adding 9.0 mL of 0.10 M HCl to 1.0 L of pure water. O Na+ buffer pH: buffer pH change: water pH change: units unitsarrow_forward
- Amoxicillin/clavulanate potassium (AUGMENTIN) powder for oral suspension is prepared prior to dispensing by adding 134 mL of purified water to the contents of the container to prepare 150 mL of suspension. If each teaspoonful of suspension contains 125 mg of amoxicillin and 31.25 mg of clavulanate potassium, how much of each of these agents is contained in the dry powder prior to reconstitutionarrow_forwardi) Write down the equation derived from your Excel generated standard curve , figure legend and describe its components; ii) provide the values of the absorbance data of the unknown sample (do NOT show the absorbance data of the glycine standards). Show all details of the working out of your calculation. Indicate all units! Provide the answer with two decimals precisionarrow_forwardDefine the term buffer. Explain the difference between carbonic acid (H2CO3) and hydrochloric acid (HCl). Can they both be used as buffers? Why or why not.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
- Essentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:Cengage

Essentials of Pharmacology for Health Professions
Nursing
ISBN:9781305441620
Author:WOODROW
Publisher:Cengage
