
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 19.8, Problem 19.15P
Interpretation Introduction
Interpretation:
The given following conversion sequence has to be shown.
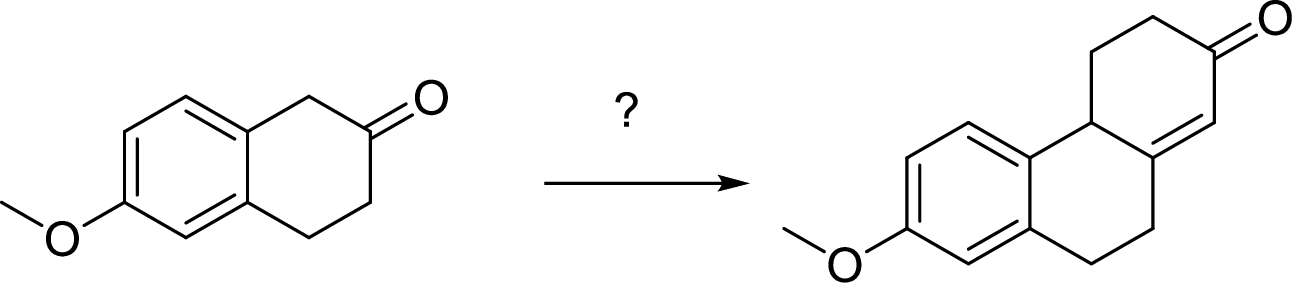
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Complete the following transformations
C10T05Q3420
Give the major organic product for the following reaction.
H
H
H
catalytic
heat
if
There is no reaction under these conditions or the correct product is not listed here.
Methyl isocyanate, CH3 -N= C = O, is used in the industrial synthesis of a type of pesticide and herbicide known as a carbamate. As a historical note, an industrial accident in Bhopal, India, in 1984 resulted in leakage of an unknown quantity of this chemical into the air. An estimated 200,000 people were exposed to its vapors, and over 2000 of these people died.
Q.) Methyl isocyanate reacts with strong acids, such as sulfuric acid, to form a cation. Will this molecule undergo protonation more readily on its oxygen or nitrogen atom? In considering contributing structures to each hybrid, do not consider structures in which more than one atom has an incomplete octet
Chapter 19 Solutions
Organic Chemistry
Ch. 19.2 - Prob. 19.1PCh. 19.2 - Prob. 19.2PCh. 19.2 - Prob. 19.3PCh. 19.3 - Prob. 19.4PCh. 19.3 - Prob. 19.5PCh. 19.3 - Prob. 19.6PCh. 19.5 - Prob. 19.7PCh. 19.5 - Prob. 19.8PCh. 19.5 - Prob. 19.9PCh. 19.6 - Prob. 19.10P
Ch. 19.6 - Prob. 19.11PCh. 19.7 - Prob. 19.12PCh. 19.8 - Prob. 19.13PCh. 19.8 - Prob. 19.14PCh. 19.8 - Prob. 19.15PCh. 19.8 - Prob. 19.16PCh. 19.9 - Prob. 19.17PCh. 19.9 - Prob. AQCh. 19.9 - Prob. BQCh. 19.9 - Prob. CQCh. 19.9 - Prob. DQCh. 19.9 - Prob. EQCh. 19.9 - Prob. FQCh. 19.9 - Prob. GQCh. 19.9 - Intermediate G in Synthesis III is produced as a...Ch. 19.9 - Prob. IQCh. 19.9 - Prob. JQCh. 19 - Prob. 19.18PCh. 19 - Prob. 19.19PCh. 19 - Prob. 19.20PCh. 19 - Prob. 19.21PCh. 19 - Prob. 19.22PCh. 19 - Prob. 19.23PCh. 19 - Cyclohexene can be converted to...Ch. 19 - Prob. 19.25PCh. 19 - Prob. 19.26PCh. 19 - Prob. 19.27PCh. 19 - Prob. 19.28PCh. 19 - Prob. 19.29PCh. 19 - Prob. 19.30PCh. 19 - Draw structural formulas for the -ketoesters...Ch. 19 - Prob. 19.32PCh. 19 - Prob. 19.33PCh. 19 - Propose a synthesis for each ketone, using as one...Ch. 19 - Prob. 19.35PCh. 19 - Claisen condensation between diethyl phthalate and...Ch. 19 - Prob. 19.37PCh. 19 - Prob. 19.38PCh. 19 - Prob. 19.39PCh. 19 - Enamines normally react with methyl iodide to give...Ch. 19 - Prob. 19.41PCh. 19 - Prob. 19.42PCh. 19 - Prob. 19.43PCh. 19 - Prob. 19.44PCh. 19 - Prob. 19.45PCh. 19 - Prob. 19.46PCh. 19 - Prob. 19.47PCh. 19 - Prob. 19.48PCh. 19 - Prob. 19.49PCh. 19 - Prob. 19.50PCh. 19 - Prob. 19.51PCh. 19 - Prob. 19.52PCh. 19 - Show experimental conditions by which to carry out...Ch. 19 - Prob. 19.55PCh. 19 - The compound 3,5,5-trimethyl-2-cyclohexenone can...Ch. 19 - Prob. 19.57PCh. 19 - Prob. 19.58PCh. 19 - The widely used anticoagulant warfarin (see...Ch. 19 - Following is a retrosynthetic analysis for an...Ch. 19 - Following are the steps in one of the several...Ch. 19 - Prob. 19.62PCh. 19 - Prob. 19.63PCh. 19 - Prob. 19.65PCh. 19 - Prob. 19.67PCh. 19 - Prob. 19.68PCh. 19 - Prob. 19.69PCh. 19 - In Problem 7.28, we saw this two-step sequence in...Ch. 19 - Using your reaction roadmaps as a guide, show how...Ch. 19 - Using your reaction roadmaps as a guide, show how...Ch. 19 - Using your reaction roadmaps as a guide, show how...Ch. 19 - Using your reaction roadmaps as a guide, show how...Ch. 19 - Using your reaction roadmaps as a guide, show how...Ch. 19 - Prob. 19.79PCh. 19 - Prob. 19.80PCh. 19 - Prob. 19.81PCh. 19 - The following molecule undergoes an intramolecular...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4. What are the products from the following reaction? H3O+ کاarrow_forwardAn important step in one synthesis of carboxylic acids is the deprotonation of diethyl malonate and its alkyl-substituted derivative: Base CH;CH2O OCH,CH3 CH;CH,0 OCH2CH3 H2 Diethyl malonate Base CH;CH,0 °C `OCH,CH3 CH;CH,O OCH,CH3 R Alkyl substituted diethyl malonate NaOH can deprotonate diethyl malonate effectively, but NaOC(CH3)3 is typically used to deprotonate the alkyl-substituted derivative. Explain why.arrow_forwardA Q2 Provide products. Br₂ AgNO3 H₂0arrow_forward
- Draw structures for compounds A through D. 1. NaOH, H2O A 2. H,0°A 1. NaOEt, EtOH (a) Ethyl acetoacetate → B 2. 1-Chloro-4-methylpentane Same as above 1. NaOH, H2O D 1. NaOC(CH3)3, (CH3);COH (b) A 2. 1-Chloropropane 2. H,0ºAarrow_forwardcompound H CH, — N — С — СН — СН, || amide - CH, — CH, — с — СH, - - CH3 – CH = CH - CHarrow_forwardc) rite in the reagent(s) over the arrow. a) C6H5N₂+ b) C6H5C=N H3C- An OH H₂C → benzene H3C benzylamine CI CH3arrow_forward
- Provide reactants, reagents, and/or products for the followin NH2 + Harrow_forwardANIC SYNTHESIS: C-CEC-C Nata ccccc 2 3. - - - C ا) c-c- de Br. C Br c-et-c Brot السلام ے کہ 2 - OHow Carrow_forwardWrite down the common (not IUPAC) names of the organic molecules that would be released if this molecule were hydrolyzed: CH2−O−C—(CH2);–CH=CH–CH2–CH=CH—(CH2)4—CH3 CH-O-C-(CH2)14-CH3 O 11 CH2−O−C— (CH2)14 — CH3 Separate each name with a comma. You will find useful information in the ALEKS Data resource. 1 010 Continue O a X 000 Y F8 F9 Submiarrow_forward
- 3-Methyl-2-hexenoic acid (mixture of E and Z isomers) has been identified as the substance responsible for the odor of human sweat. Synthesize the compound from raw materials that have five carbons or less.arrow_forwardWhat is the major product of the following reaction? ||| ا IV I & III NaOH مداد III IVarrow_forwardC15T05Q2545 Give the major product(s) of the following reaction. CH3CH2CI CI AICI3, heat CI CI CI CI- CI- CI There is no reaction under these conditions or the correct product is not listed here.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY