
When methyl red is added to an aqueous solution, a pink color results. When methyl orange is added to the same solution, a yellow color is produced. What is the approximate
Interpretation:
The
Concept introduction:
Acid-base indicator is a chemical which changes its color on neutralization.
There are various acid-base indicators like phenolphthalein, methyl orange, bromocresol green, bromocresol purple, phenol red
All have a specific
Answer to Problem 89A
Explanation of Solution
The diagram below shows that methyl orange changes its color from pink to yellow close to
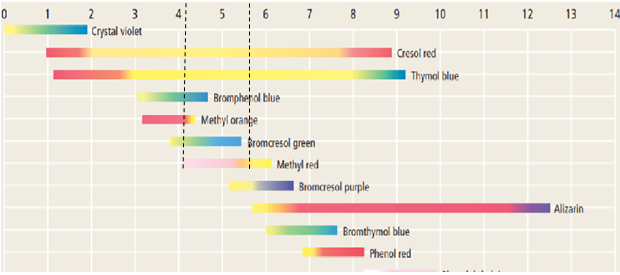
Thus, the given solution has
Chapter 18 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Biological Science (6th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Microbiology: An Introduction
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Anatomy & Physiology (6th Edition)
Chemistry: Structure and Properties (2nd Edition)
- (f) SO: Best Lewis Structure 3 e group geometry:_ shape/molecular geometry:, (g) CF2CF2 Best Lewis Structure polarity: e group arrangement:_ shape/molecular geometry: (h) (NH4)2SO4 Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward1. Problem Set 3b Chem 141 For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the molecule is polar or non-polar (iv) (a) SeF4 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: (b) AsOBr3 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles):arrow_forward(c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





