
Concept explainers
Interpretation: The correct sequence of three consecutive steps which constitutes the hydrolysis of an imine/iminium has to be determined.
Concept introduction:
Amino acids are the molecules containing an
Carbon, hydrogen, oxygen and nitrogen are the key elements in amino acid.
General structure of an amino acid can be drawn as follows,
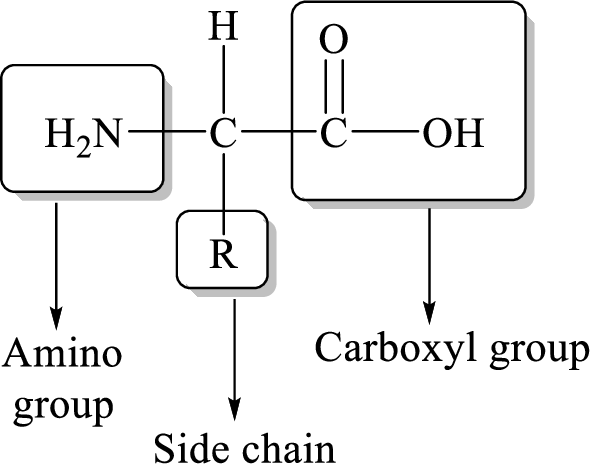
The transfer of an amino group from an amino acid to a ketoacid with the formation of a new amino acid and a new keto acid is known as transamination reaction.
This reaction is catalysed by a group of enzymes called transaminases or aminotransferases. PLP- Pyridoxalphosphate acts as co-factor in this reaction.
Imines can be obtained by the reaction of an
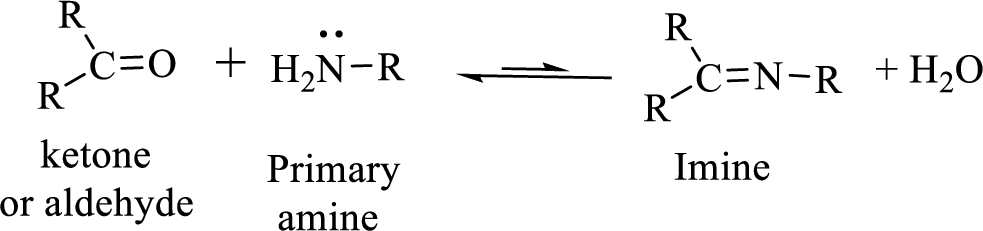
Simple imines hydrolyze back to the aldehyde or ketone and the amine from which they are originally formed.
Iminium ions on hydrolysis give corresponding amine and ketoacid.
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Organic Chemistry
- Definition and classification of boranes.arrow_forwardWhich of the terms explain the relationship between the two compounds? CH2OH Он Он Он Он α-D-galactose anomers enantiomers diastereomers epimers CH2OH ОН O он Он ОН B-D-galactosearrow_forwardHi, I need help on my practice final, If you could offer strategies and dumb it down for me with an explanation on how to solve that would be amazing and beneficial.arrow_forward
- Hi I need help with my practice final, it would be really helpful to offer strategies on how to solve it, dumb it down, and a detailed explanation on how to approach future similar problems like this. The devil is in the details and this would be extremely helpfularrow_forwardIn alpha-NbI4, Nb4+ should have the d1 configuration (bond with paired electrons: paramagnetic). Please comment.arrow_forwardHi, I need help on my practice final, if you could explain how to solve it offer strategies and dumb it down that would be amazing. Detail helpsarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning



