
(a)
Interpretation:
The given reactions are final steps in one industrial synthesis of vitamin A acetate.
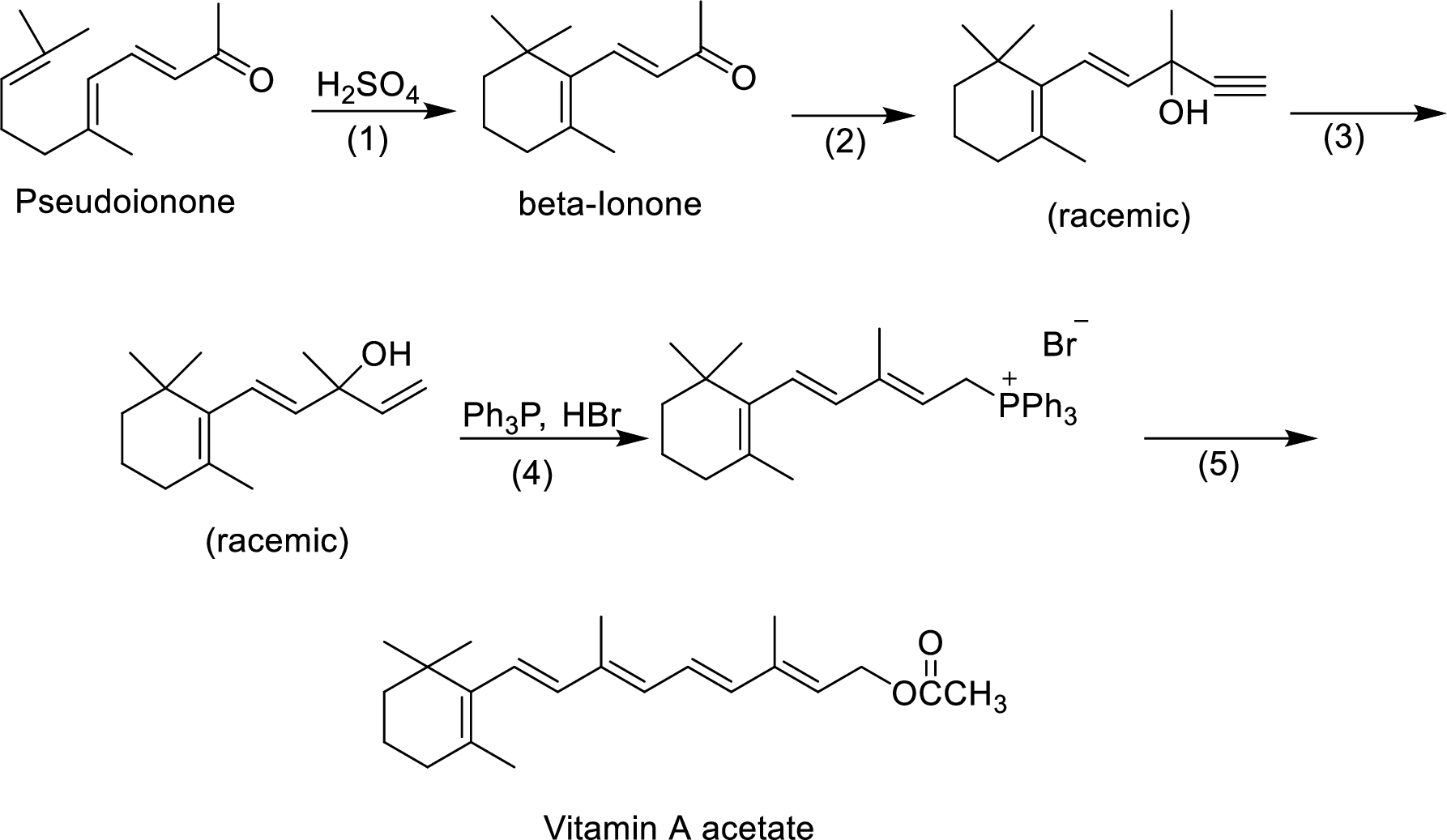
The mechanism has to be proposed for the acid-catalyzed cyclization in step-1.
Concept Introduction:
The ring cyclization occurs when an acyclic molecule reacts with acid. by the abstraction of proton from the acid molecule, internal bond shifting occurs to form the cyclization product.
(b)
Interpretation:
The given reactions are final steps in one industrial synthesis of vitamin A acetate.
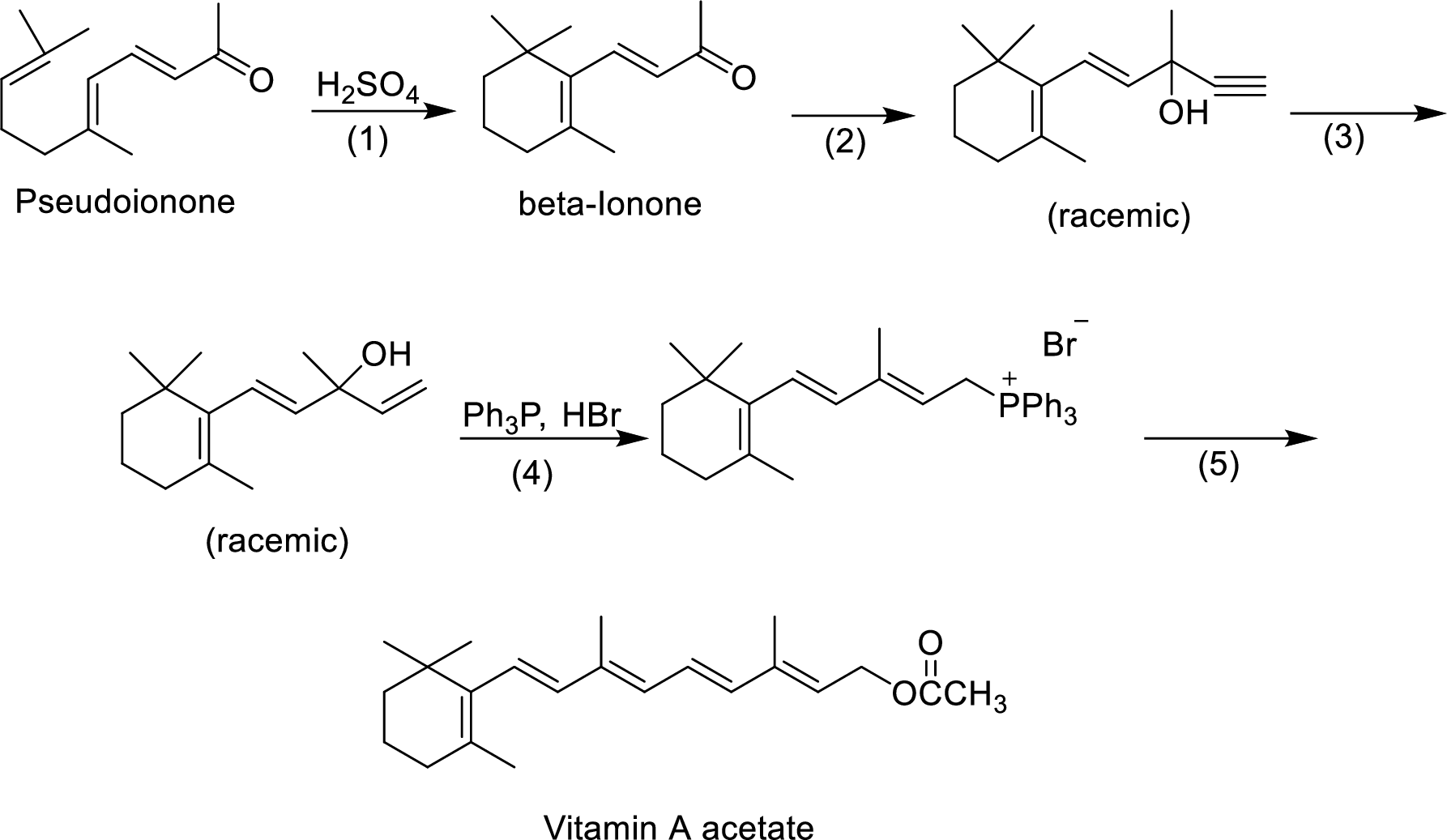
The reagents has to be given for the reaction involved in step-2.
(c)
Interpretation:
The given reactions are final steps in one industrial synthesis of vitamin A acetate.
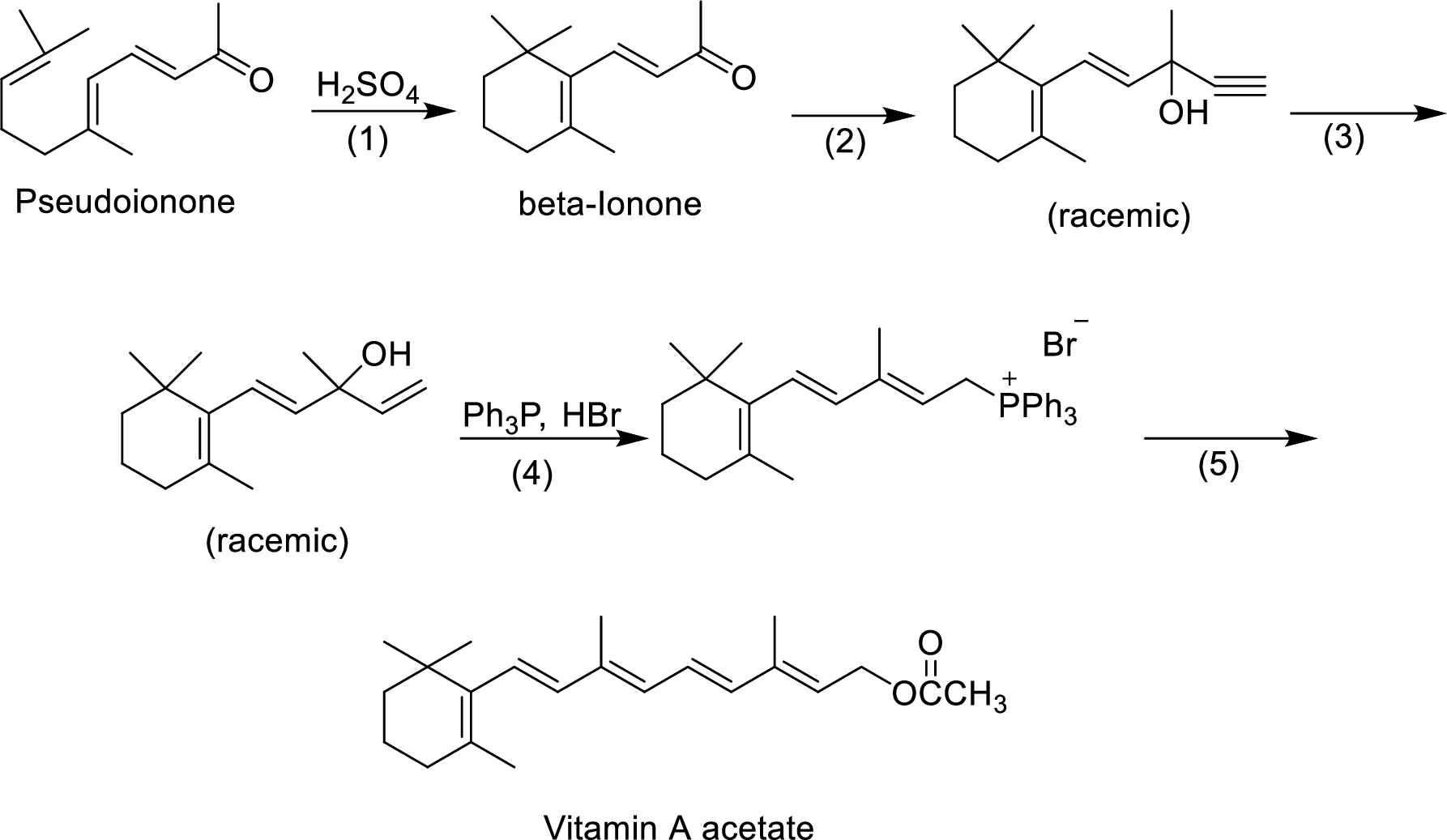
The mechanism has to be proposed for the formation of phosphonium salt in step-4.
(d)
Interpretation:
The given reactions are final steps in one industrial synthesis of vitamin A acetate.
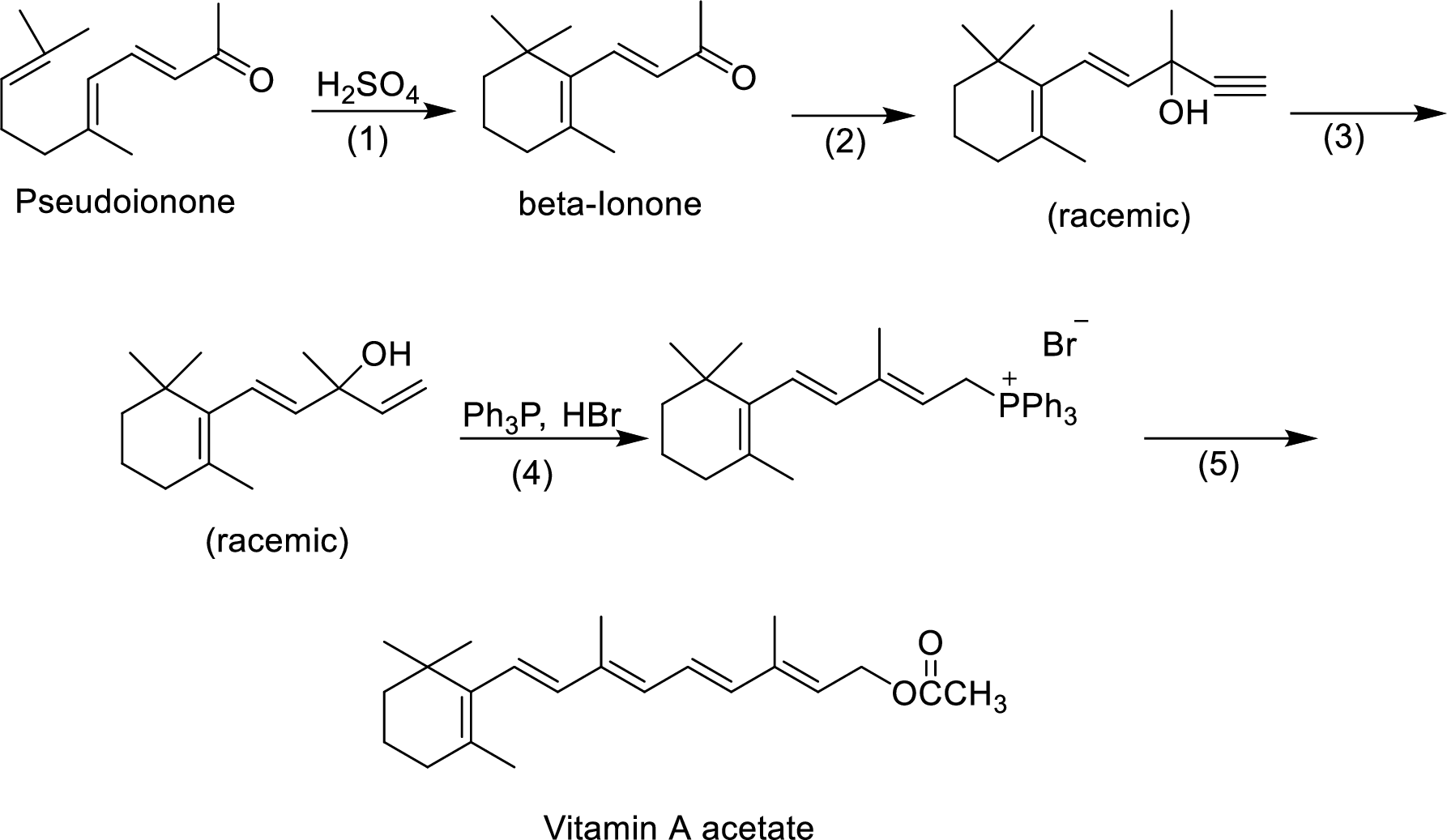
The reagents that are involved in the reaction of step-3 has to be proposed.
(e)
Interpretation:
The given reactions are final steps in one industrial synthesis of vitamin A acetate.
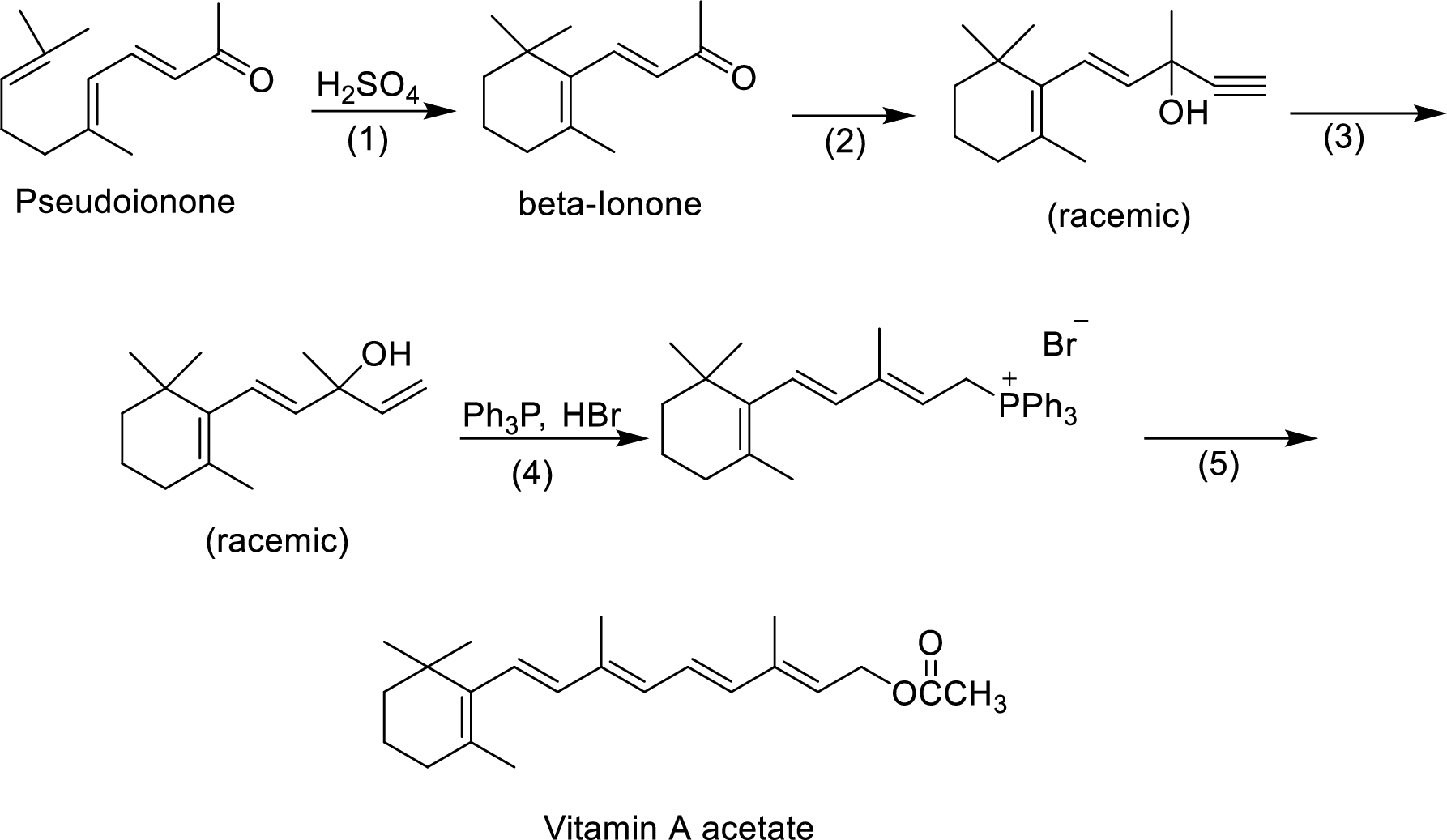
Wittig reaction of step-5 has to be shown.
Concept Introduction:
Wittig reaction:
The reaction of carbonyl compound with the ylide of phosphonium salt to give an
Trending nowThis is a popular solution!

Chapter 16 Solutions
Organic Chemistry
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

