
Interpretation:
The difference between an intermediate and an activated complex needs to be explained.
Concept introduction:
Answer to Problem 37SSC
| Activated complex | Intermediate |
| Highly unstable | Less unstable than activated complex |
| Cannot isolate | Can isolate |
| Very high energy | High energy than reactant and product |
| Example: Activated complex | Example: Carbocation, carbanion, carbene |
Explanation of Solution
The reaction sequence of a multi-step reaction which defines the complete conversion reactant to product molecules is called as reaction mechanism. The active species which are formed during reaction mechanism and are readily converted to product are called as intermediate. Once the reactant molecules acquire activation energy, they form activated complex which is highly unstable due to highest energy and readily converts to product.
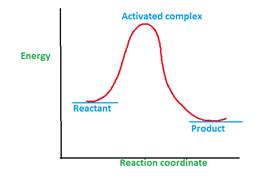
Thus the major difference between activated complex and intermediate is:
| Activated complex | Intermediate |
| Highly unstable | Less unstable than activated complex |
| Cannot isolate | Can isolate |
| Very high energy | High energy than reactant and product |
| Example: Activated complex | Example: Carbocation, carbanion, carbene |
Activated complex are unstable species whereas intermediates are more stable than activated complex.
Chapter 16 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
General, Organic, and Biological Chemistry (3rd Edition)
Chemistry: Structure and Properties (2nd Edition)
Chemistry: The Central Science (14th Edition)
Organic Chemistry (8th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





