
Interpretation:
A diagram needs to be constructed that shows all of the possible collision combinations between two molecules of Reactant A and two molecules of Reactant B. Also, each possible A-B collision combination needs to be sketched on increasing the number of molecules A from two to four. The factor increasing the number of collision combinations needs to be explained.
Concept introduction:
Molecules undergo collision in the presence of the required amount of energy needed for collision to form some new molecules as well as in the presence of correct molecular orientation.
Answer to Problem 87A
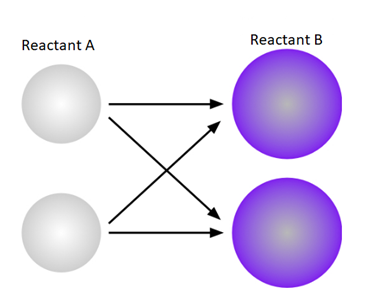
The above diagram shows the possible collision between two molecules A and B. There is a possibility of four collisions in this case.
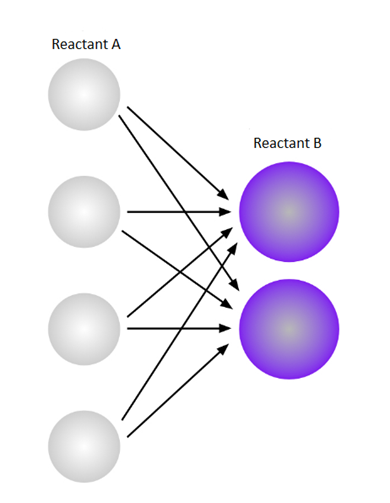
The above diagram shows possible collision when the number of reactant A is double. The possible collision in this case is 8.
The reaction rate doubles when the reactant molecule taken is doubled.
Explanation of Solution
A diagram that shows all of the possible collision combinations between two molecules of Reactant A and two molecules of Reactant B is given below.
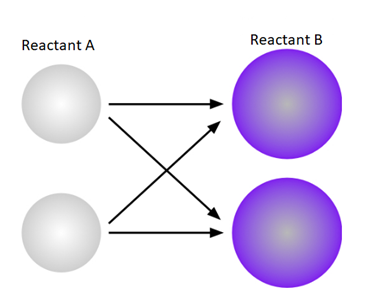
There four different possible combinations are possible on collision of two molecules of reactant A and two molecules of reactant B.
Now, a diagram where each possible A-B collision combination on increasing the number of molecules A from two to four is shown below,
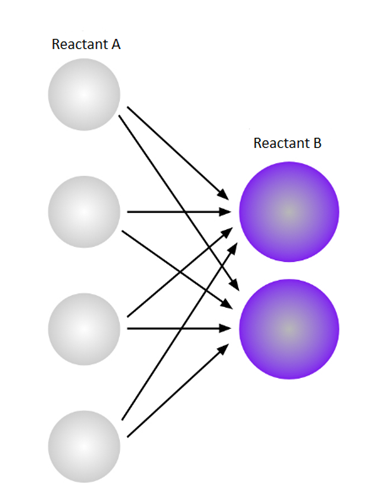
On increasing the number of moleculeA from two to four, there exist 8 possible combinations of the different reactants during a collision. By this we can say that on doubling the concentration of one reactant, the number of possible collisions also doubles. The doubling in the collision, the reaction rate also tends to double.
The reaction rate doubles when the reactant molecule taken is doubled.
Chapter 16 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Chemistry: Structure and Properties (2nd Edition)
Introductory Chemistry (6th Edition)
Campbell Biology: Concepts & Connections (9th Edition)
Cosmic Perspective Fundamentals
Applications and Investigations in Earth Science (9th Edition)
Microbiology: An Introduction
- Show work. Don't give Ai and copied solutionarrow_forwardNonearrow_forwardUnshared, or lone, electron pairs play an important role in determining the chemical and physical properties of organic compounds. Thus, it is important to know which atoms carry unshared pairs. Use the structural formulas below to determine the number of unshared pairs at each designated atom. Be sure your answers are consistent with the formal charges on the formulas. CH. H₂ fo H2 H The number of unshared pairs at atom a is The number of unshared pairs at atom b is The number of unshared pairs at atom c is HC HC HC CH The number of unshared pairs at atom a is The number of unshared pairs at atom b is The number of unshared pairs at atom c isarrow_forward
- Draw curved arrows for the following reaction step. Arrow-pushing Instructions CH3 CH3 H H-O-H +/ H3C-C+ H3C-C-0: CH3 CH3 Harrow_forward1:14 PM Fri 20 Dec 67% Grade 7 CBE 03/12/2024 (OOW_7D 2024-25 Ms Sunita Harikesh) Activity Hi, Nimish. When you submit this form, the owner will see your name and email address. Teams Assignments * Required Camera Calendar Files ... More Skill: Advanced or complex data representation or interpretation. Vidya lit a candle and covered it with a glass. The candle burned for some time and then went off. She wanted to check whether the length of the candle would affect the time for which it burns. She performed the experiment again after changing something. Which of these would be the correct experimental setup for her to use? * (1 Point) She wanted to check whether the length of the candle would affect the time for which it burns. She performed the experiment again after changing something. Which of these would be the correct experimental setup for her to use? A Longer candle; No glass C B Longer candle; Longer glass D D B Longer candle; Same glass Same candle; Longer glassarrow_forwardBriefly describe the compounds called carboranes.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





