
Interpretation:
An advertisement explaining that company A’s fertilizer works better than company B’s fertilizer because it has smaller sized granules needs to be represented.
Concept introduction:
Fertilizers are used in plants or soil to supply the essential nutrients of them. They are used to increase the productivity of crops. The fertilizers contain the essential nutrients which are needed by the plants like nitrogen, potassium and phosphorus.
Answer to Problem 99A
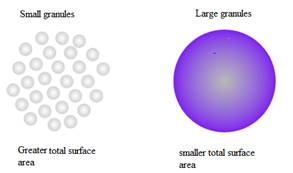
As shown in the figure below that larger granule due to their larger size they have smaller surface area more of the chemicals would be locked away in the middle of the granules that wouldn’t be able to interact with the soil. But the small granules have greater surface area therefore, the more the chemicals in the fertilizer are exposed to the soil around it and can react with it.
Explanation of Solution
Smaller granules are easier to use in fertilizer as the granules in the bag with smaller pieces potentially add up to a more visible surface area than the bag of larger granules for the same amount of a bag of fertilizer.
The higher the fertilizer's surface area has, the more chemicals in the fertilizer are exposed to and can react with, the soil around it. If the granules were bigger, some of the contaminants that could not interact with the soil would be locked away in the center of the granules. So, buy company A’s fertilizer for quicker and more crop production.
Smaller granule fertilizers are easy to use than larger granule fertilizer because larger size granule takes more space than smaller size granule fertilizers.
Chapter 16 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Microbiology: An Introduction
Cosmic Perspective Fundamentals
Human Anatomy & Physiology (2nd Edition)
Introductory Chemistry (6th Edition)
Biology: Life on Earth (11th Edition)
Chemistry: The Central Science (14th Edition)
- A gas following mole compositions at 120 \deg F, 13.8 psia. N2% 2, CH 4% 79C2H6 % 19. Volume fractionn?arrow_forwardPlease correct answer and don't used hand raitingarrow_forwardOrder-disorder phenomenaa) do not have conductive properties.b) are cooperative.c) have few industrial implications.arrow_forward
- Unshared, or lone, electron pairs play an important role in determining the chemical and physical properties of organic compounds. Thus, it is important to know which atoms carry unshared pairs. Use the structural formulas below to determine the number of unshared pairs at each designated atom. Be sure your answers are consistent with the formal charges on the formulas. CH. H₂ fo H2 H The number of unshared pairs at atom a is The number of unshared pairs at atom b is The number of unshared pairs at atom c is HC HC HC CH The number of unshared pairs at atom a is The number of unshared pairs at atom b is The number of unshared pairs at atom c isarrow_forwardDraw curved arrows for the following reaction step. Arrow-pushing Instructions CH3 CH3 H H-O-H +/ H3C-C+ H3C-C-0: CH3 CH3 Harrow_forward1:14 PM Fri 20 Dec 67% Grade 7 CBE 03/12/2024 (OOW_7D 2024-25 Ms Sunita Harikesh) Activity Hi, Nimish. When you submit this form, the owner will see your name and email address. Teams Assignments * Required Camera Calendar Files ... More Skill: Advanced or complex data representation or interpretation. Vidya lit a candle and covered it with a glass. The candle burned for some time and then went off. She wanted to check whether the length of the candle would affect the time for which it burns. She performed the experiment again after changing something. Which of these would be the correct experimental setup for her to use? * (1 Point) She wanted to check whether the length of the candle would affect the time for which it burns. She performed the experiment again after changing something. Which of these would be the correct experimental setup for her to use? A Longer candle; No glass C B Longer candle; Longer glass D D B Longer candle; Same glass Same candle; Longer glassarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





