
(a)
Interpretation: The electron dot formula of
Concept Introduction: Using a "dot formula," valence electrons are shown as a group of dots encircling the element symbol.
(a)
Answer to Problem 91A
The electron dot formula of
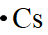
Explanation of Solution
In the periodic table of elements, cesium is placed in group one, which indicates that it contains one valence electron.
The electron dot structure of
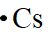
(b)
Interpretation: The electron dot formula of
Concept Introduction: Using a "dot formula," valence electrons are shown as a group of dots encircling the element symbol.
(b)
Answer to Problem 91A
The electron dot formula of
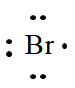
Explanation of Solution
In the periodic table of elements, bromine is placed in group seven, which indicates that it contains seven valence electrons.
The electron dot structure of
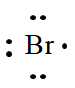
(c)
Interpretation: The electron dot formula of
Concept Introduction: Using a "dot formula," valence electrons are shown as a group of dots encircling the element symbol.
(c)
Answer to Problem 91A
The electron dot formula of
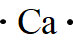
Explanation of Solution
In the periodic table of elements, calcium is placed in group two, which indicates that it contains two valence electrons.
The electron dot structure of
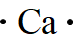
(d)
Interpretation: The electron dot formula of
Concept Introduction: Using a "dot formula," valence electrons are shown as a group of dots encircling the element symbol..
(d)
Answer to Problem 91A
The electron dot formula of
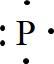
Explanation of Solution
In the periodic table of elements, phosphorus is placed in group five, which indicates that it contains five valence electrons.
The electron dot structure of
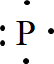
Chapter 12 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





