
(a) Interpretation:
The balanced equation for the reaction catalyzed by PFK-2 should be written.
Concept Introduction:
PFK-2 is an enzyme that indirectly regulates the glycolysis rate in the cells. It is known to be a bifunctional enzyme due to its structure. The two domains independently act as functional enzymes because both are located on one protein honodimer.
In mammals, different PFK-2 isoforms are encoded by genetic mechanisms to accommodate tissue specific needs. General function of PFK-2 remains the same. Slight differences in enzymatic properties are featured by isoforms which are then controlled by different methods of regulation.
(b) Interpretation:
The balanced equation for the conversion of 2 moles of oxaloacetate to glucose should be written.
Concept Introduction:
Gluconeogenesis is a
(c) Interpretation:
The balanced equation for the conversion of glucose to UDP-Glc should be written.
Concept Introduction:
ATP to ADP conversion is reduction reaction. Energy released from the hydrolysis of ATP to ADP, this energy is used in cellular processes. Also, ADP can combined with a phosphate to form ATP, the reaction is as follows:
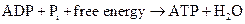
(d) Interpretation:
The balanced equation for the conversion of 2 moles of glycerol to glucose should be written.
Concept Introduction:
ATP to ADP conversion is reduction reaction. Energy released from the hydrolysis of ATP to ADP, this energy is used in cellular processes. Also, ADP can combine with a phosphate to form ATP, the reaction is as follows:
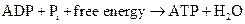
Also, NAD+ is an oxidizing agent as it accepts electrons from other molecules to get reduced to NADH. Here, NADH can act as a reducing agent to donate electrons. Thus, the main function of NAD is electron transfer reaction.
(e) Interpretation:
The balanced equation for the conversion of 2 moles of malate to glucose-6-phosphate should be written.
Concept Introduction:
ATP to ADP conversion is reduction reaction. Energy released from the hydrolysis of ATP to ADP, this energy is used in cellular processes. Also, ADP can combine with a phosphate to form ATP, the reaction is as follows:
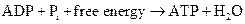
Similarly, the conversion of GTP to GDP is also a reduction reaction.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Biochemistry: Concepts and Connections (2nd Edition)
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





