
(a)
Interpretation:
The electronic configuration which is likely to form negative ion needs to be determined.
Concept Introduction:
An element that by accepting electron will achieve a half-filled or full-filled electronic configuration will likely to form a negative ion.
(a)
Answer to Problem 74A
Explanation of Solution
If the
(b)
Interpretation:
The electronic configuration which is likely to have low ionization energy needs to be determined.
Concept Introduction:
Removal of an electron that will give the most stable half-filled and full-filled electronic configuration will have the lowest ionization energy.
(b)
Answer to Problem 74A
Explanation of Solution
When an electron is removed from
Now,
(c)
Interpretation:
The most reactive metal is to be identified.
Concept Introduction:
Metals which easily donate electrons to achieve stable half-filled or full-filled configuration are most reactive. Alkali metals are reactive due to their electronic configuration.
(c)
Answer to Problem 74A
Explanation of Solution
If the
Now,
(d)
Interpretation:
The electronic configuration which is likely to have more unpaired of electrons needs to be determined.
Concept Introduction:
When an electron does not get paired up in orbital but occupies the orbital singly then it is called an unpaired electron.
(d)
Answer to Problem 74A
Explanation of Solution
Orbital diagram of
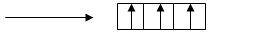
It is obvious from the diagram that there are three unpaired electrons which is highest among the options given.
(e)
Interpretation:
The metal that will combine with oxygen in
Concept Introduction:
A metal which can donate two electrons from its valence to complete the octet of oxygen will combine with oxygen in
(e)
Answer to Problem 74A
Explanation of Solution
Magnesium (Mg) will donate two electrons from its
(f)
Interpretation:
The formula the compound formed between
Concept Introduction:
A stable compound is formed when both the atoms participating in the combination achieves complete octet.
(f)
Answer to Problem 74A
The formula of the compound is
Explanation of Solution
So, 3
(g)
Interpretation:
The formula the compound formed between
Concept Introduction:
A stable compound is formed when both the atoms participating achieves complete octet.
(g)
Answer to Problem 74A
The formula of the compound is
Explanation of Solution
Three
(h)
Interpretation:
The order of ionization energy needs to be determined.
Concept Introduction:
Ionization energy is a measure of the difficulty in removing an electron from an atom or ion or the tendency of an atom or ion to lose an electron. It is the energy required. So, this process is endothermic.
(h)
Answer to Problem 74A
Order of increase in ionization energy is
Explanation of Solution
- The ionization energy of the elements usually increases throughout the period.
- The ionization energy of the elements usually decreases throughout the group.
- Ionization energies of noble gases are very high because of octet completion. That is why neon has the highest ionization energy of all the elements given.
Considering all facts, the order of increasing ionization energy is
Chapter 11 Solutions
World of Chemistry, 3rd edition
- 5. A solution of sucrose is fermented in a vessel until the evolution of CO2 ceases. Then, the product solution is analyzed and found to contain, 45% ethanol; 5% acetic acid; and 15% glycerin by weight. If the original charge is 500 kg, evaluate; e. The ratio of sucrose to water in the original charge (wt/wt). f. Moles of CO2 evolved. g. Maximum possible amount of ethanol that could be formed. h. Conversion efficiency. i. Per cent excess of excess reactant. Reactions: Inversion reaction: C12H22O11 + H2O →2C6H12O6 Fermentation reaction: C6H12O6 →→2C2H5OH + 2CO2 Formation of acetic acid and glycerin: C6H12O6 + C2H5OH + H₂O→ CH3COOH + 2C3H8O3arrow_forwardShow work. don't give Ai generated solution. How many carbons and hydrogens are in the structure?arrow_forward13. (11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the molecule depicted below. Bond B 2°C. +2°C. cleavage Bond A •CH3 + 26.← Cleavage 2°C. + Bond C +3°C• CH3 2C Cleavage E 2°C. 26. weakest bond Intact molecule Strongest 3°C 20. Gund Largest argest a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in appropriate boxes. C Weakest bond A Produces Most Bond Strongest Bond Strongest Gund produces least stable radicals Weakest Stable radical b. (4pts) Consider the relative stability of all cleavage products that form when bonds A, B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B, and C are all carbon radicals. i. Which ONE cleavage product is the most stable? A condensed or bond line representation is fine. 13°C. formed in bound C cleavage ii. Which ONE cleavage product is the least stable? A condensed or bond line representation is fine. • CH3 methyl radical Formed in Gund A Cleavage c.…arrow_forward
- Hi!! Please provide a solution that is handwritten. Ensure all figures, reaction mechanisms (with arrows and lone pairs please!!), and structures are clearly drawn to illustrate the synthesis of the product as per the standards of a third year organic chemistry course. ****the solution must include all steps, mechanisms, and intermediate structures as required. Please hand-draw the mechanisms and structures to support your explanation. Don’t give me AI-generated diagrams or text-based explanations, no wordy explanations on how to draw the structures I need help with the exact mechanism hand drawn by you!!! I am reposting this—ensure all parts of the question are straightforward and clear or please let another expert handle it thanks!!arrow_forwardHi!! Please provide a solution that is handwritten. Ensure all figures, reaction mechanisms (with arrows and lone pairs please!!), and structures are clearly drawn to illustrate the synthesis of the product as per the standards of a third year organic chemistry course. ****the solution must include all steps, mechanisms, and intermediate structures as required. Please hand-draw the mechanisms and structures to support your explanation. Don’t give me AI-generated diagrams or text-based explanations, no wordy explanations on how to draw the structures I need help with the exact mechanism hand drawn by you!!! I am reposting this—ensure all parts of the question are straightforward and clear or please let another expert handle it thanks!!arrow_forward. (11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the molecule depicted below. Bond B 2°C. +2°C. < cleavage Bond A • CH3 + 26. t cleavage 2°C• +3°C• Bond C Cleavage CH3 ZC '2°C. 26. E Strongest 3°C. 2C. Gund Largest BDE weakest bond In that molecule a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in appropriate boxes. Weakest C bond Produces A Weakest Bond Most Strongest Bond Stable radical Strongest Gund produces least stable radicals b. (4pts) Consider the relative stability of all cleavage products that form when bonds A, B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B, and C are all carbon radicals. i. Which ONE cleavage product is the most stable? A condensed or bond line representation is fine. 人 8°C. formed in bound C cleavage ii. Which ONE cleavage product is the least stable? A condensed or bond line representation is fine. methyl radical •CH3 formed in bund A Cleavagearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





