
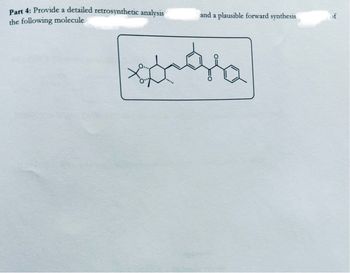
Hi!!
Please provide a solution that is handwritten. Ensure all figures, reaction mechanisms (with arrows and lone pairs please!!), and structures are clearly drawn to illustrate the synthesis of the product as per the standards of a third year
Please hand-draw the mechanisms and structures to support your explanation. Don’t give me AI-generated diagrams or text-based explanations, no wordy explanations on how to draw the structures I need help with the exact mechanism hand drawn by you!!!
I am reposting this—ensure all parts of the question are straightforward and clear or please let another expert handle it thanks!!
Step by stepSolved in 2 steps with 2 images

- When propenal is treated with sodium acetylide, a product is formed whose IR spectrum exhibits a broad absorption between 3200 and 3600 cm¯1, but shows no absorption near 1700 cm-1. (a) Draw the structure of the product. (b) Argue whether the nucleophile adds reversibly or irreversibly to the carbonyl group. CH HC || CH2 1. HC=CNa ? 2. NH,CIarrow_forwardGive detailed Solution with explanation neededarrow_forwardGive detailed Solution with explanationarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

