
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
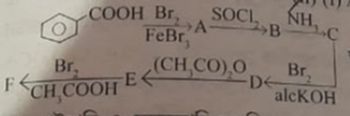
Transcribed Image Text:Br.
COOH Br,
FCH COOH E
FeBr
ASOCI
B
NH
(CH,CO),OD Br₂
2
C
alcKOH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- 25. Write reasonable mechanisms for each of the following transformations.arrow_forward4) H3C Br Br CH3 'CI + + HỈOH Н ОН Дон ОН SH КОН H2Oarrow_forwardWhich of the following gases consist of molecules containing four or more atoms? Rn O NO O CO Ar Kr CO2 Хе H,S HF H, F, N, sO, Ne HCN Cl, NO, CH, Не Sa HCI MacBook Proarrow_forward
- Write a reasonable chain mechanism (mechanistic arrows, intermediates, etc.) for the following reaction. Your mechanism must be based upon currently accepted understanding of chemical reactions.arrow_forward11. Me2NH, CH,O HCI Me dr Mearrow_forwardR Please don't provide the handwriting solutionarrow_forward
- 30. The process for preparing dibromoethane is given in the reaction equation below. Using the bond energies listed, determine the enthalpy change (AH) for this reaction. H H нн + Brz(2) Br-С - С-Br e AH = ? kJ c=C@ нн нн Bond Bond Energy (kJ/mol) С-Н 411 C=C 602 Br-Br 190 С-Br 285 C-C 346 | Select ) [ Select + 36 kJ -124 kJ + 124 kJ + 78 kJ - 78 kJarrow_forward2. Use the table below to estimate AH for the reaction NO + O3 → NO2 + O2. Does this reaction consume or produce heat? Single Bond Energies (kJ/mol of bonds) н сN oS F C Br I Н 436 C 413 346 N 391 305 163 O 463 358 201 146 S 347 272 226 - F 565 485 283 190 284 155 Cl 432 339 192 218 255 253 242 Br 366 285 201 217 249 216 193 - I 299 213 201 278 208 175 151 - - Multiple Bond Energies (kJ/mol of bonds) C=C 602 C=N 615 C=O 799 C=C 835 CEN 887 C=O 1072 N=N 418 N=O 607 N=N 945 O=O 498arrow_forwardWhich of these three is most stable? Explain (justify)arrow_forward
- Calculate ?H° for each oxidation reaction. Each equation is balanced as written; remember to take into account thecoefficients in determining the number of bonds broken or formed.?H° for O2 = 497 kJ/mol; ?H° for one C = O in CO2 = 535 kJ/mol]a. CH4 + 2 O2 ? CO2 + 2 H2Ob. 2 CH3CH3 + 7 O2 ? 4 CO2 + 6 H2Oarrow_forwardWrite the law for this reaction and explain how it is determined SO2CL2 ->SO2 + CL2arrow_forwarddraw LINE BOND STRUCTUREarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning