
Biochemistry: The Molecular Basis of Life
6th Edition
ISBN: 9780190209896
Author: Trudy McKee, James R. McKee
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 3, Problem 2Q
Summary Introduction
To review:
The non-covalent bonds responsible for the interactionsindicated by the shaded regions in the given proteins.
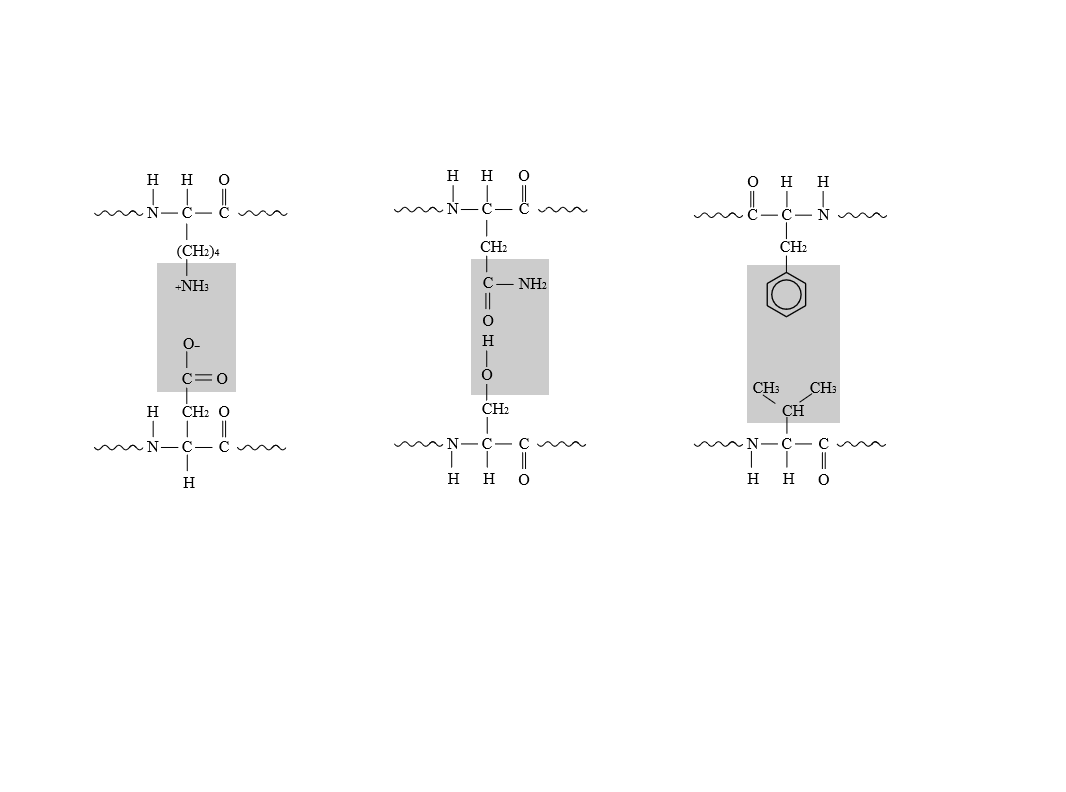
Introduction:
Proteins are
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
All proteins have primary (1°), secondary (2°) and tertiary (3°) structure. In addition, some (but not all) proteins exhibit quaternary (4°) structure. Explain quaternary structure and why only some proteins exhibit that level of structure.
Hydrogen bonds and hydrochloric interactions play important roles in stabilizing and organizing biological macromolecules. Describe how Hydrogen bonds and hydrochloric interactions affects the form and function of proteins.
At what level of protein structure (primary, secondary, tertiary or quarternary) does Hydrogen bonding is relevant ? Consider if there is no Hydrogen bonding that exists, and only van der Waals exists in this protein structure, what do you expect to happen in its property? Explain with examples.
Chapter 3 Solutions
Biochemistry: The Molecular Basis of Life
Ch. 3 - Prob. 1QCh. 3 - Prob. 2QCh. 3 - Prob. 3QCh. 3 - Prob. 4QCh. 3 - Prob. 1RQCh. 3 - Prob. 2RQCh. 3 - Prob. 3RQCh. 3 - Prob. 4RQCh. 3 - Prob. 5RQCh. 3 - Prob. 6RQ
Ch. 3 - Prob. 7RQCh. 3 - Prob. 8RQCh. 3 - Prob. 9RQCh. 3 - Prob. 10RQCh. 3 - Prob. 11RQCh. 3 - Prob. 12RQCh. 3 - Prob. 13RQCh. 3 - Prob. 14RQCh. 3 - Prob. 15RQCh. 3 - Prob. 16RQCh. 3 - Prob. 17RQCh. 3 - Prob. 18RQCh. 3 - Prob. 19RQCh. 3 - Prob. 20RQCh. 3 - Prob. 21RQCh. 3 - Prob. 22RQCh. 3 - Prob. 23RQCh. 3 - Prob. 24RQCh. 3 - Prob. 25RQCh. 3 - Prob. 26RQCh. 3 - Prob. 27RQCh. 3 - Prob. 28RQCh. 3 - Prob. 29RQCh. 3 - Prob. 30RQCh. 3 - Prob. 31RQCh. 3 - Prob. 32RQCh. 3 - Prob. 33RQCh. 3 - Prob. 34RQCh. 3 - Prob. 35RQCh. 3 - Prob. 36FBCh. 3 - Prob. 37FBCh. 3 - Prob. 38FBCh. 3 - Prob. 39FBCh. 3 - Prob. 40FBCh. 3 - Prob. 41FBCh. 3 - Prob. 42FBCh. 3 - Prob. 43FBCh. 3 - Prob. 44FBCh. 3 - Prob. 45FBCh. 3 - Prob. 46SACh. 3 - Prob. 47SACh. 3 - Prob. 48SACh. 3 - Prob. 49SACh. 3 - Prob. 50SACh. 3 - Prob. 51TQCh. 3 - Prob. 52TQCh. 3 - Prob. 53TQCh. 3 - Prob. 54TQCh. 3 - Prob. 55TQCh. 3 - Prob. 56TQCh. 3 - Prob. 57TQCh. 3 - Prob. 58TQCh. 3 - Prob. 59TQCh. 3 - Prob. 60TQCh. 3 - Prob. 61TQCh. 3 - Prob. 62TQCh. 3 - Prob. 63TQCh. 3 - Prob. 64TQCh. 3 - Prob. 65TQCh. 3 - Prob. 66TQCh. 3 - Prob. 67TQCh. 3 - Prob. 68TQCh. 3 - Prob. 69TQCh. 3 - Prob. 70TQCh. 3 - Prob. 71TQCh. 3 - Prob. 72TQCh. 3 - Prob. 73TQCh. 3 - Prob. 74TQCh. 3 - Prob. 75TQCh. 3 - Prob. 76TQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- In general, proteins can be classified into 3 different groups. Name and give a short description of each type and how they are distinct from one another. Provide an example of macromolecule or other complex structure representing each of the three types.arrow_forwardCarbon’s versatile bonding behavior allows it to form a variety of structures and are the base of all macromolecules. The diagram shows the structure of an amino acid, which is the monomer unit for proteins. Identify and describe the polymer structures of a protein that constitutes its unique conformation.arrow_forwardDraw the chemical structure of an alanine pentapeptide. Indicate the location of each peptide bond. Label the phi () and psi () dihedral angles. Name and briefly define the four levels of protein structure.arrow_forward
- Why is the 3-Dimensional structure important for protein function? What factors or agents can denature protein structure? Give examples (more than one factor) Why denaturation affect the function of proteins? Explain the structure - function relationship.arrow_forwardList and discuss the types of interactions that stabilize the tertiary structure of proteins. For each stabilizing interaction, identify one example of denaturant and describe how the interactions are disrupted.arrow_forwardT/F: The titration curve for serine will have 2 inflection points. T/F: The Amino Acid D-selenocysteine has an R-Configuration T/F: Each water molecule can form H bonds with 4 other water molecules T/F: In a buffer system, decreasing the concentration of the conjugate base relative to acid makes the buffer more acidic T/F: Glycine forms good interactions in a protein structure True or false?arrow_forward
- Identify the following statements as descriptive of the secondary, tertiary, or quaternary structure of a protein. What types of interactions stabilize each type of structure?(a) The polypeptide chain has a number of bends and twists, resulting in a compact structure.(b) The polypeptide backbone forms a right-handed coil.(c) The four polypeptide chains are arranged in a spherical shape.arrow_forwardGlycine provides structural flexibility in proteins. What is the consequence of this on protein structure?arrow_forwardHow do the following interactions help to stabilize the tertiary and quaternary structure of a protein? Give an example of a pair of amino acids that could give rise to each interaction.(a) Side-chain hydrogen bonding(b) Disulfide bondsarrow_forward
- Basic chemical properties of polar positively charged amino acids in proteins. Use a few examples.arrow_forwardhow does the protein environment surrounding an amino acid chain affect its chemical properties?Consider the carboxyl group on an asparate side chain in the following environments in a protein. Rank in order these environments from the highest to the lowest proportion of carboxyl groups in the -COO- form.that is in terms of pKas. 1. an aspartate side chain on the surface of protein with no other ionizable groups nearby. 2. an aspartate side chain buried in a hydrophobic pocket on the surface of a protein 3. an asparate chain in a hydrophobic pocket adjacent to a glutamte side chain 4. an asparate side chain in a hydrophobic porket adjacent to a lysine side chain.arrow_forwardDescribe the forces that are involved in the tertiary structure of a protein and give an example of each force listed.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON
Macromolecules | Classes and Functions; Author: 2 Minute Classroom;https://www.youtube.com/watch?v=V5hhrDFo8Vk;License: Standard youtube license