
Concept explainers
Interpretation:
The total energy required to break all the bonds in 1 mole of ethanol needs to be determined.
Concept introduction:
The bond energy of a chemical bond can be defined as the energy required to break that chemical bond. The bond energy that is needed to break the bonds in reactant molecule and the energy released to form
Thus, the sum of bond energies of all bonds in a molecule will be the energy which requires to break all bonds.
Answer to Problem 13STP
Option (C) is correct answer.
Explanation of Solution
Reason for correct option:
Structure of ethanol can be shown as:
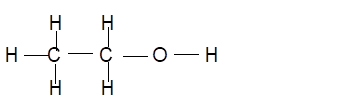
Total energy required to break all bonds:
Reasons for incorrect options: The sum of bond energies of all chemical bonds present in a molecule is the energy required forbreaking all the bonds,therefore,option (b), (c) and (d) are incorrect.
Chapter U5 Solutions
Living by Chemistry
Additional Science Textbook Solutions
Campbell Biology (11th Edition)
Biology: Life on Earth (11th Edition)
Campbell Essential Biology (7th Edition)
Chemistry: Structure and Properties (2nd Edition)
Chemistry (7th Edition)
Organic Chemistry (8th Edition)
- Five chemistry project topic that does not involve practicalarrow_forwardPlease correct answer and don't used hand raitingarrow_forwardQ2. Consider the hydrogenation of ethylene C2H4 + H2 = C2H6 The heats of combustion and molar entropies for the three gases at 298 K are given by: C2H4 C2H6 H2 AH comb/kJ mol¹ -1395 -1550 -243 Sº / J K¹ mol-1 220.7 230.4 131.1 The average heat capacity change, ACP, for the reaction over the temperature range 298-1000 K is 10.9 J K¹ mol¹. Using these data, determine: (a) the standard enthalpy change at 800 K (b) the standard entropy change at 800 K (c) the equilibrium constant at 800 K.arrow_forward
- 13. (11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the molecule depicted below. Bond B Bond A Bond C a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in appropriate boxes. Weakest Bond Strongest Bond b. (4pts) Consider the relative stability of all cleavage products that form when bonds A, B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B, and C are all carbon radicals. i. Which ONE cleavage product is the most stable? A condensed or bond line representation is fine. ii. Which ONE cleavage product is the least stable? A condensed or bond line representation is fine. c. (5pts) Use principles discussed in lecture, supported by relevant structures, to succinctly explain the why your part b (i) radical is more stable than your part b(ii) radical. Written explanation can be no more than one-two succinct sentence(s)! Googlearrow_forwardPrint Last Name, First Name Initial Statifically more chances to abstract one of these 6H 11. (10pts total) Consider the radical chlorination of 1,3-diethylcyclohexane depicted below. 4 4th total • 6H total 래 • 4H total 21 total ZH 2H Statistical H < 3° C-H weakest - product abstraction here bund leads to thermo favored a) (6pts) How many unique mono-chlorinated products can be formed and what are the structures for the thermodynamically and statistically favored products? Product 6 Number of Unique Mono-Chlorinated Products Thermodynamically Favored Product Statistically Favored Product b) (4pts) Draw the arrow pushing mechanism for the FIRST propagation step (p-1) for the formation of the thermodynamically favored product. Only draw the p-1 step. You do not need to include lone pairs of electrons. No enthalpy calculation necessary H H-Cl Waterfoxarrow_forward10. (5pts) Provide the complete arrow pushing mechanism for the chemical transformation → depicted below Use proper curved arrow notation that explicitly illustrates all bonds being broken, and all bonds formed in the transformation. Also, be sure to include all lone pairs and formal charges on all atoms involved in the flow of electrons. CH3O II HA H CH3O-H H ①arrow_forward
- Do the Lone Pairs get added bc its valence e's are a total of 6 for oxygen and that completes it or due to other reasons. How do we know the particular indication of such.arrow_forwardNGLISH b) Identify the bonds present in the molecule drawn (s) above. (break) State the function of the following equipments found in laboratory. Omka) a) Gas mask b) Fire extinguisher c) Safety glasses 4. 60cm³ of oxygen gas diffused through a porous hole in 50 seconds. How long w 80cm³ of sulphur(IV) oxide to diffuse through the same hole under the same conditions (S-32.0.0-16.0) (3 m 5. In an experiment, a piece of magnesium ribbon was cleaned with steel w clean magnesium ribbon was placed in a crucible and completely burnt in oxy cooling the product weighed 4.0g a) Explain why it is necessary to clean magnesium ribbon. Masterclass Holiday assignmen PB 2arrow_forwardHi!! Please provide a solution that is handwritten. Ensure all figures, reaction mechanisms (with arrows and lone pairs please!!), and structures are clearly drawn to illustrate the synthesis of the product as per the standards of a third year organic chemistry course. ****the solution must include all steps, mechanisms, and intermediate structures as required. Please hand-draw the mechanisms and structures to support your explanation. Don’t give me AI-generated diagrams or text-based explanations, no wordy explanations on how to draw the structures I need help with the exact mechanism hand drawn by you!!! I am reposting this—ensure all parts of the question are straightforward and clear or please let another expert handle it thanks!!arrow_forward
- In three dimensions, explain the concept of the velocity distribution function of particles within the kinetic theory of gases.arrow_forwardIn the kinetic theory of gases, explain the concept of the velocity distribution function of particles in space.arrow_forwardIn the kinetic theory of gases, explain the concept of the velocity distribution function of particles.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





