
Concept explainers
Interpretation: The following bonds needs to be placed in order of increasing energy released when the bond is formed.
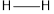



Concept introduction: Bond energy is measured as the amount of the energy needed to break a bond like that of carbon-hydrogen bond. It is the measure of the strength of the particular bond.
Answer to Problem 4E
The bonds in order of increasing energy released when the bond is formed is,

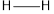


Explanation of Solution
The average bond energy of is 413,
is 413, 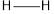 is 432,
is 432,  is 467 and
is 467 and  799.
799.
Therefore, the bonds in order of increasing energy released when the bond is formed is,

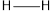


Considering the average bond energy, the bonds in order of increasing energy released when the bond is formed is,

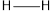


Chapter U5 Solutions
Living by Chemistry
Additional Science Textbook Solutions
Chemistry
General, Organic, and Biological Chemistry (3rd Edition)
Introductory Chemistry (6th Edition)
Chemistry For Changing Times (14th Edition)
Chemistry: The Central Science (13th Edition)
Organic Chemistry (9th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





