
(a)
Interpretation:
Molecular formula of hexanol must be given.
Concept introduction:
Molecular formula is the general formula of the molecule which shows the number of different atoms.
Answer to Problem 5E
Molecular formula of hexanol is C6H14O.
Explanation of Solution
Aliphatic alcohol has general formula CnH2n + 2O. As in hexanol there are 6 carbon atoms, so total number of hydrogen must be 14. There is one O atom which is present in the hydroxyl (alcoholic OH)
(b)
Interpretation:
A possible structural formula of hexanol must be drawn.
Concept introduction:
Structural formula is the representation of a molecule in which the arrangements of all the atoms are shown.
Answer to Problem 5E
Structural formula of hexanol is shown below.
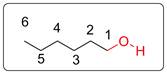
Explanation of Solution
One possible structural formula of hexanol acid is drawn in which all the carbon atoms have 4 bonds. The O has two bonds and two lone pairs of electrons. There is no lone pair on C and H atoms. Hydrogen atoms of the carbon chain are not shown to avoid complexity of the structure.
Many hexanol possibly depend on the position of functional (alcoholic OH) group.
(c)
Interpretation:
Whether molecular formula or structural formula is more important in determining the smell of the compound must be explained.
Concept introduction:
Structural formula gives more information regarding three dimensional arrangements of atoms and shape of a molecule whereas molecular formula only gives number of different atoms.
Answer to Problem 5E
Structural formula is more important in determining the smell of a compound.
Explanation of Solution
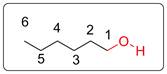
The functional group (OH) present in hexanol is shown in red. The functional group is present in carbon-1. The structure of the hexanol will change if the OH group is present in other carbons. Thus, to get the actual shape of the molecule, structural formula is essential. Many structures can be drawn for a single molecular formula. So, to determine the smell of a compound, the idea of shape of the molecule is important. Thus, structural formula is more important than molecular formula in determining the smell of a molecule.
Chapter U2 Solutions
Living By Chemistry: First Edition Textbook
Additional Science Textbook Solutions
Campbell Biology: Concepts & Connections (9th Edition)
Campbell Biology (11th Edition)
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Microbiology: An Introduction
Microbiology: An Introduction
Campbell Biology in Focus (2nd Edition)
- 5. A solution of sucrose is fermented in a vessel until the evolution of CO2 ceases. Then, the product solution is analyzed and found to contain, 45% ethanol; 5% acetic acid; and 15% glycerin by weight. If the original charge is 500 kg, evaluate; e. The ratio of sucrose to water in the original charge (wt/wt). f. Moles of CO2 evolved. g. Maximum possible amount of ethanol that could be formed. h. Conversion efficiency. i. Per cent excess of excess reactant. Reactions: Inversion reaction: C12H22O11 + H2O →2C6H12O6 Fermentation reaction: C6H12O6 →→2C2H5OH + 2CO2 Formation of acetic acid and glycerin: C6H12O6 + C2H5OH + H₂O→ CH3COOH + 2C3H8O3arrow_forwardShow work. don't give Ai generated solution. How many carbons and hydrogens are in the structure?arrow_forward13. (11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the molecule depicted below. Bond B 2°C. +2°C. cleavage Bond A •CH3 + 26.← Cleavage 2°C. + Bond C +3°C• CH3 2C Cleavage E 2°C. 26. weakest bond Intact molecule Strongest 3°C 20. Gund Largest argest a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in appropriate boxes. C Weakest bond A Produces Most Bond Strongest Bond Strongest Gund produces least stable radicals Weakest Stable radical b. (4pts) Consider the relative stability of all cleavage products that form when bonds A, B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B, and C are all carbon radicals. i. Which ONE cleavage product is the most stable? A condensed or bond line representation is fine. 13°C. formed in bound C cleavage ii. Which ONE cleavage product is the least stable? A condensed or bond line representation is fine. • CH3 methyl radical Formed in Gund A Cleavage c.…arrow_forward
- Hi!! Please provide a solution that is handwritten. Ensure all figures, reaction mechanisms (with arrows and lone pairs please!!), and structures are clearly drawn to illustrate the synthesis of the product as per the standards of a third year organic chemistry course. ****the solution must include all steps, mechanisms, and intermediate structures as required. Please hand-draw the mechanisms and structures to support your explanation. Don’t give me AI-generated diagrams or text-based explanations, no wordy explanations on how to draw the structures I need help with the exact mechanism hand drawn by you!!! I am reposting this—ensure all parts of the question are straightforward and clear or please let another expert handle it thanks!!arrow_forwardHi!! Please provide a solution that is handwritten. Ensure all figures, reaction mechanisms (with arrows and lone pairs please!!), and structures are clearly drawn to illustrate the synthesis of the product as per the standards of a third year organic chemistry course. ****the solution must include all steps, mechanisms, and intermediate structures as required. Please hand-draw the mechanisms and structures to support your explanation. Don’t give me AI-generated diagrams or text-based explanations, no wordy explanations on how to draw the structures I need help with the exact mechanism hand drawn by you!!! I am reposting this—ensure all parts of the question are straightforward and clear or please let another expert handle it thanks!!arrow_forward. (11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the molecule depicted below. Bond B 2°C. +2°C. < cleavage Bond A • CH3 + 26. t cleavage 2°C• +3°C• Bond C Cleavage CH3 ZC '2°C. 26. E Strongest 3°C. 2C. Gund Largest BDE weakest bond In that molecule a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in appropriate boxes. Weakest C bond Produces A Weakest Bond Most Strongest Bond Stable radical Strongest Gund produces least stable radicals b. (4pts) Consider the relative stability of all cleavage products that form when bonds A, B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B, and C are all carbon radicals. i. Which ONE cleavage product is the most stable? A condensed or bond line representation is fine. 人 8°C. formed in bound C cleavage ii. Which ONE cleavage product is the least stable? A condensed or bond line representation is fine. methyl radical •CH3 formed in bund A Cleavagearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





