
Concept explainers
(a)
Interpretation: The number of lone pairs in TeCl2 molecule needs to be determined.
Concept Introduction:
Lewis dot structure or electron dot structure can be defined as the structure of a molecule in which all the valence electrons and lone pairs on each atom is represented with the help of line and dot or cross.
The Lewis structure is used to predict the hybridization and molecular geometry of the molecule as it gives complete idea about the bonding between bonded atoms in a molecule.
(a)
Answer to Problem 6E
8 lone pairs; 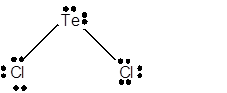
Explanation of Solution
The number of lone pairs in any molecule can be determined with the help of Lewis structure. In the Lewis structure of TeCl2 molecule;
Number of valence electrons in Te = 6 electrons
Number of valence electrons in Cl = 7
Total number of valence electrons = 6 + (2 x 7) = 20 electrons.
Thus the Lewis structure must be:
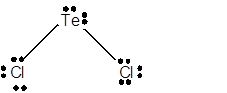
Hence in TeCl2 molecule there are 8 lone pairs.
(b)
Interpretation: The number of lone pairs in HI molecule needs to be determined.
Concept Introduction:
Lewis dot structure or electron dot structure can be defined as the structure of a molecule in which all the valence electrons and lone pairs on each atom is represented with the help of line and dot or cross.
The Lewis structure is used to predict the hybridization and molecular geometry of the molecule as it gives complete idea about the bonding between bonded atoms in a molecule.
(b)
Answer to Problem 6E
3 lone pairs; 
Explanation of Solution
The number of lone pairs in any molecule can be determined with the help of Lewis structure. In the Lewis structure of HI molecule;
Number of valence electrons in I = 7 electrons
Number of valence electrons in H= 1
Total number of valence electrons = 1 + (1 x 7) = 8 electrons.
Thus the Lewis structure must be:

Hence in HI molecule there are 3 lone pairs.
(c)
Interpretation: The number of lone pairs in AsBr3molecule needs to be determined.
Concept Introduction:
Lewis dot structure or electron dot structure can be defined as the structure of a molecule in which all the valence electrons and lone pairs on each atom is represented with the help of line and dot or cross.
The Lewis structure is used to predict the hybridization and molecular geometry of the molecule as it gives complete idea about the bonding between bonded atoms in a molecule.
(c)
Answer to Problem 6E
10 lone pairs; 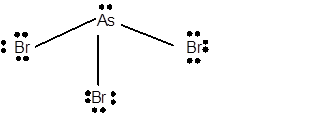
Explanation of Solution
The number of lone pairs in any molecule can be determined with the help of Lewis structure. In the Lewis structure of AsBr3 molecule;
Number of valence electrons in As = 5 electrons
Number of valence electrons in Br = 7
Total number of valence electrons = 5 + (3 x 7) = 26 electrons.
Thus the Lewis structure must be:
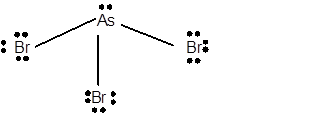
Hence in AsBr3 molecule there are 10 lone pairs.
(d)
Interpretation: The number of lone pairs in SiF4 molecule needs to be determined.
Concept Introduction:
Lewis dot structure or electron dot structure can be defined as the structure of a molecule in which all the valence electrons and lone pairs on each atom is represented with the help of line and dot or cross.
The Lewis structure is used to predict the hybridization and molecular geometry of the molecule as it gives complete idea about the bonding between bonded atoms in a molecule.
(d)
Answer to Problem 6E
12 lone pairs; 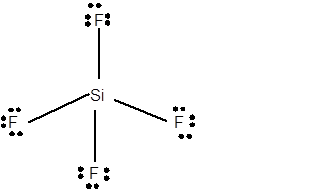
Explanation of Solution
The number of lone pairs in any molecule can be determined with the help of Lewis structure. In the Lewis structure of SiF4 molecule;
Number of valence electrons in Si = 4 electrons
Number of valence electrons in F = 7
Total number of valence electrons = 4 + (4 x 7) = 32 electrons.
Thus the Lewis structure must be:
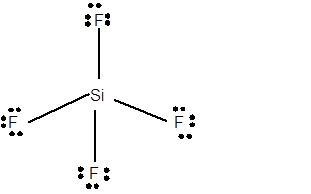
Hence in SiF4 moleculethere are 12 lone pairs.
(e)
Interpretation: The number of lone pairs in F2 molecule needs to be determined.
Concept Introduction:
Lewis dot structure or electron dot structure can be defined as the structure of a molecule in which all the valence electrons and lone pairs on each atom is represented with the help of line and dot or cross.
The Lewis structure is used to predict the hybridization and molecular geometry of the molecule as it gives complete idea about the bonding between bonded atoms in a molecule.
(e)
Answer to Problem 6E
6 lone pairs; 
Explanation of Solution
The number of lone pairs in any molecule can be determined with the help of Lewis structure. In the Lewis structure of F2 molecule;
Number of valence electrons in F = 7
Total number of valence electrons = (2x 7) = 14 electrons.
Thus the Lewis structure must be:

Hence in F2 molecule there are 6 lone pairs.
Chapter U2 Solutions
Living By Chemistry: First Edition Textbook
Additional Science Textbook Solutions
Campbell Biology (11th Edition)
Chemistry (7th Edition)
Chemistry: A Molecular Approach (4th Edition)
Introductory Chemistry (6th Edition)
Applications and Investigations in Earth Science (9th Edition)
Brock Biology of Microorganisms (15th Edition)
- Don't used hand raiting and don't used Ai solutionarrow_forward2' P17E.6 The oxidation of NO to NO 2 2 NO(g) + O2(g) → 2NO2(g), proceeds by the following mechanism: NO + NO → N₂O₂ k₁ N2O2 NO NO K = N2O2 + O2 → NO2 + NO₂ Ко Verify that application of the steady-state approximation to the intermediate N2O2 results in the rate law d[NO₂] _ 2kk₁[NO][O₂] = dt k+k₁₂[O₂]arrow_forwardPLEASE ANSWER BOTH i) and ii) !!!!arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





