
Concept explainers
(a)
Interpretation :
Shape of ammonia molecule must be predicted.
Concept Introduction :
Shape of a molecule is determined by the total number of electron pairs in the valence shell of the central atom.
Ammonia molecule has total 4 atoms in which N is the central atom.
(a)
Answer to Problem SII2RE
Shape of ammonia molecule is trigonal pyramidal.
Explanation of Solution
The structure of ammonia molecule is represented as follows:

N has total 4 pairs of electrons in the valence shell.
There are three bond pairs and lone pairs of electrons. N atom is sp3 hybridized and thus NH3 has trigonal pyramidal shape due to presence of one lone pair electrons.
b)
Interpretation :
Shape of silicon tetrachloride must be predicted.
Concept Introduction :
Shape of a molecule is determined by the total number of electron pairs in the valence shell of the central atom.
Silicon tetrachloride molecule has total 5 atoms in which Si is the central atom.
b)
Answer to Problem SII2RE
Shape of silicon tetrachloride molecule is tetrahedral.
Explanation of Solution
The structure is represented as follows:
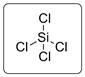
Si has total 4 pairs of electrons in the valence shell.
There are four bond pairs and no lone pairs of electrons. Si atom is sp3 hybridized and thus SiCl4 has tetrahedral shape.
c)
Interpretation :
Shape of hydrogen sulfide must be predicted.
Concept Introduction :
Shape of a molecule is determined by the total number of electron pairs in the valence shell of the central atom.
Hydrogen sulfide molecule has total 3 atoms in which S is the central atom.
c)
Answer to Problem SII2RE
Shape of hydrogen molecule is angular.
Explanation of Solution
The structure of H2S is represented as follows:
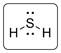
S has total 4 pairs of electrons in the valence shell.
There are two bond pairs and two lone pairs of electrons. S atom is sp3 hybridized and thus H2S has angular shape.
d)
Interpretation :
Shape of hydrogen cyanide must be predicted.
Concept Introduction :
Shape of a molecule is determined by the total number of electron pairs in the valence shell of the central atom.
Hydrogen cyanide molecule has total 3 atoms in which C is the central atom.
d)
Answer to Problem SII2RE
Shape of hydrogen cyanide is linear.
Explanation of Solution
The structure is represented as follows:
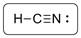
C has total 4 pairs of electrons in the valence shell.
There are four bond pairs and no lone pairs of electrons. C atom is triple bonded with N and carbon is sp hybridized and thus HCN has linear shape.
e)
Interpretation :
Shape of formaldehyde must be predicted.
Concept Introduction :
Shape of a molecule is determined by the total number of electron pairs in the valence shell of the central atom.
Formaldehyde molecule has total 4 atoms in which C is the central atom.
e)
Answer to Problem SII2RE
Shape of formaldehyde is trigonal planar.
Explanation of Solution
The structure of formaldehyde is represented as follows:
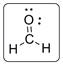
C has total 4 pairs of electrons in the valence shell.
There are four bond pairs and no lone pairs of electrons. C atom is double bonded with O and single bonded with two hydrogen atoms. Carbon is sp2 hybridized and thus CH2N has trigonal planar shape.
Chapter U2 Solutions
Living By Chemistry: First Edition Textbook
Additional Science Textbook Solutions
Campbell Biology (11th Edition)
Introductory Chemistry (6th Edition)
Applications and Investigations in Earth Science (9th Edition)
Campbell Biology in Focus (2nd Edition)
Microbiology with Diseases by Body System (5th Edition)
Organic Chemistry (8th Edition)
- Macmillan Learning Draw the acyl chloride that would give the ketone shown using the Friedel-Crafts acylation reaction. Select Draw Templates More с H о Cl 2Q Erase AICI₂arrow_forwardDraw the complete mechanism for this reaction: .OH مدید OH H2SO4 + H₂O To save you some time, the starting material has been copied into the first drawing area. However, you will still need to add any other reactants or catalysts that take part in the reaction. ན ི.. OH Add/Remove step Х ด ك Click and drag to start drawing a structure.arrow_forward9:27 AM Tue Mar 4 ← Problem 64 of 15 #63% Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. 0:0 0:0 :0: N. :0: :O :0: H H. :0: Select to Add Arrows O :0: H O :0: 0:0. S. H Select to Add Arrows S :0: :0: H Harrow_forward
- Order the following organic reactions by relative rate. That is, select '1' next to the reaction that will have the fastest initial rate, select '2' next to the reaction that will have the next fastest initial rate, and so on. If two reactions will have very similar initial rates, you can select the same number next to both. If a reaction will have zero or nearly zero initial rate, don't select a number and check the box in the table instead. Note: the "Nu" in these reactions means "a generic nucleophile." ملی CI :Nu 2 он 3 H Reaction Relative Rate (Choose one) ▼ Nu :CI: zero or nearly zero Nu :Nu bi (Choose one) zero or nearly zero : Nu لی Nu :H (Choose one) zero or nearly zeroarrow_forward9:12 AM Tue Mar 4 66% Problem 38 of 15 Submit Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the product formed in this reaction or mechanistic step(s). Include all lone pairs and charges as appropriate. Ignore inorganic byproducts. Br2 FeBrз H (+) Br: H : Br----FeBr3 く a SU 00 nd earrow_forwardUnder aqueous acidic conditions, nitriles will react to form a neutral organic intermediate 1 that has an N atom in it first, and then they will continue to react to form the final product 2: ☐ : P Draw the missing intermediate 1 and the final product 2 in the box below. You can draw the two structures in any arrangement you like. CN H₂O H₂O H+ H+ Click and drag to start drawing a structure. Хarrow_forward
- Organic bases have lone pairs of electrons that are capable of accepting protons. Lone pair electrons in a neutral or negatively charged species, or pi electron pairs. Explain the latter case (pi electron pairs).arrow_forwardDescribe the propyl anion.arrow_forwardIndicate the names of these compounds (if they exist). 0: HỌC—NH CH3CH2-CH2arrow_forward
- N Classify each of the following molecules as aromatic, antiaromatic, or nonaromatic. NH O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic O nonaromatic Garrow_forwardThe conjugate base of alkanes is called alkides. Correct?.arrow_forwardName these organic compounds: structure Br name CH3 CH3 ☐ ☐arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





