
Concept explainers
Write Lewis dot symbols for the following atoms and ions:
Interpretation:
The Lewis dot symbols for the given atoms and ions are to be written.
Concept introduction:
Lewis dot symbols contain dots that give information about the valence electrons.
Lewis dot symbols show both bond and lone pairs as dots.
Lewis structures show bonds as lines and lone pairs as dots.
Answer to Problem 5QP
Solution:
a)
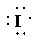
b)
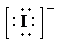
c)
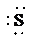
d)
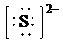
e)
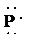
f)
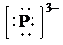
g)
h)
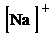
i)
j)
k)
l)
m)
n)
Explanation of Solution
a) Atom—
The number of valence electrons in
atom is
So, there are
So, the Lewis structure for
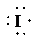
b) Ion—
The number of valence electrons in
So, there are
So, the Lewis structure for
is as follows:
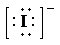
c) Atom—
The number of valence electrons in
atom is
So, there are 3 lone pairs present.
So, the Lewis structure for
is as follows:
d) Ion—
The number of valence electrons in
So, there are
So, the Lewis structure for
is as follows:
e) Atom—
The number of valence electrons in
atom is
So, there are
So, the Lewis structure for
is as follows:
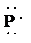
f) Ion—
The number of valence electrons in
So, there are
So, the Lewis structure for
is as follows:
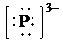
g) Atom—
The number of valence electrons in
atom is
So, there are no lone pairs present.
So, the Lewis structure for
is as follows:
h) Ion—
The number of valence electrons in
So, the Lewis structure for
is as follows:
i) Atom—
The number of valence electrons in
atom is
So, the Lewis structure for
is as follows:
j) Ion—
The number of valence electrons in
So, the Lewis structure for
is as follows:
k) Atom—
The number of valence electrons in
atom is
So, the Lewis structure for
is as follows:
l) Ion—
The number of valence electrons in
So, the Lewis structure for
is as follows:
m) Atom—
The number of valence electrons in
atom is
So, the Lewis structure for
is as follows:
n) Ion—
The number of valence electrons in
So, the Lewis structure for
is as follows:
Want to see more full solutions like this?
Chapter 8 Solutions
Chemistry
- 2B: The retrosynthetic cut below provides two options for a Suzuki coupling, provide the identities of A, B, C and D then identify which pairing is better and justify your choice. O₂N. Retro-Suzuki NO2 MeO OMe A + B OR C + Darrow_forwardthis is an organic chemistry question please answer accordindly!! please post the solution in your hand writing not an AI generated answer please draw the figures and structures if needed to support your explanation hand drawn only!!!! answer the question in a very simple and straight forward manner thanks!!!!! im reposting this please solve all parts and draw it not just word explanations!!arrow_forwardShow work. don't give Ai generated solution and don't copy answer anywherearrow_forward
- Show work. don't give Ai generated solutionarrow_forwardShow work. Don't give Ai generated solutionarrow_forwardA buffered solution containing dissolved aniline, CH,NH2, and aniline hydrochloride, CH, NH, Cl, has a pH of 5.41. Determine the concentration of CH, NH in the solution if the concentration of CH, NH, is 0.305 M. The pK of aniline is 9.13. [CHẠNH] = Calculate the change in pH of the solution, ApH, if 0.375 g NaOH is added to the buffer for a final volume of 1.40 L. Assume that any contribution of NaOH to the volume is negligible. ApH = Marrow_forward
- Electron spin magnetic moment and energy levels that occur in the presence of an external magnetic field.arrow_forwardNuclear spin energy levels and electron spin energy levels.arrow_forward1. Polyester Formation a. Draw the structure of the polyester formed (Seabacoyl Chloride + Ethylene Glycol). (Insert scanned hand-drawn structure or ChemDraw image.) b. What molecules are eliminated in this condensation reaction?arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
