
Basic Chemistry
6th Edition
ISBN: 9780134878119
Author: Timberlake, Karen C. , William
Publisher: Pearson,
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 5.1, Problem 9PP
Interpretation Introduction
Interpretation: To determine the type of
Concept introduction:
There are 7 different types of electromagnetic radiations and we can classify them based on their frequency or wavelength as:
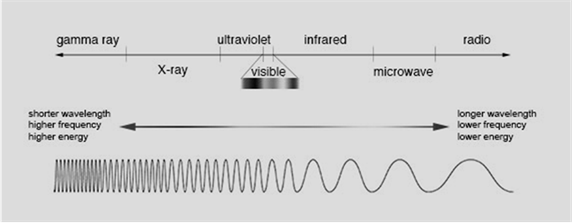
Radiations which are on left side has shorter wavelength than the radiations present in the right side.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Basic Chemistry
Ch. 5.1 - What is meant by the wavelength of UV light?Ch. 5.1 - How are the wavelength and frequency of light...Ch. 5.1 - What is the difference between "white” light and...Ch. 5.1 - Prob. 4PPCh. 5.1 - Ultraviolet radiation (UVB) used to treat...Ch. 5.1 - AM radio waves have a frequency of 8105s1, whereas...Ch. 5.1 - If orange light has a wavelength of 6105cm, what...Ch. 5.1 - A wavelength of 850 nm is used for fiber-optic...Ch. 5.1 - Prob. 9PPCh. 5.1 - Prob. 10PP
Ch. 5.1 - Prob. 11PPCh. 5.1 - Place the following types of electromagnetic...Ch. 5.1 - Prob. 13PPCh. 5.1 - Place the following types of electromagnetic...Ch. 5.2 - What feature of an atomic spectrum indicates that...Ch. 5.2 - How can we explain the distinct lines that appear...Ch. 5.2 - Prob. 17PPCh. 5.2 - Prob. 18PPCh. 5.2 - Prob. 19PPCh. 5.2 - Prob. 20PPCh. 5.3 - Describe the shape of each of the following...Ch. 5.3 - Describe the shape of each of the following...Ch. 5.3 - Match statements 1 to 3 with a to d: 1. They have...Ch. 5.3 - Match statements 1 to 3 with a to d: 1. They have...Ch. 5.3 - Prob. 25PPCh. 5.3 - Indicate the number of each in the following: a....Ch. 5.3 - Prob. 27PPCh. 5.3 - Prob. 28PPCh. 5.4 - Compare the terms electron configuration and...Ch. 5.4 - Compare the terms orbital diagram and electron...Ch. 5.4 - Draw the orbital diagram for each of the...Ch. 5.4 - Draw the orbital diagram for each of the...Ch. 5.4 - Prob. 33PPCh. 5.4 - Write the complete electron configuration for each...Ch. 5.4 - Prob. 35PPCh. 5.4 - Prob. 36PPCh. 5.4 - Prob. 37PPCh. 5.4 - Prob. 38PPCh. 5.4 - Prob. 39PPCh. 5.4 - Give the symbol of the element that meets the...Ch. 5.5 - Use the sublevel blocks on the periodic table to...Ch. 5.5 - Use the sublevel blocks on the periodic table to...Ch. 5.5 - Use the sublevel blocks on the periodic table to...Ch. 5.5 - Use the sublevel blocks on the periodic table to...Ch. 5.5 - Prob. 45PPCh. 5.5 - Use the periodic table to give the symbol of the...Ch. 5.5 - Prob. 47PPCh. 5.5 - Use the periodic table lo give the symbol of the...Ch. 5.5 - Prob. 49PPCh. 5.5 - Prob. 50PPCh. 5.6 - What do the group numbers from IA (1) to 8A (18)...Ch. 5.6 - Prob. 52PPCh. 5.6 - Write the group number using both A/B and 1 to 18...Ch. 5.6 - Write the group number using both A/B and 1 to 18...Ch. 5.6 - Write the valence electron configuration for each...Ch. 5.6 - Prob. 56PPCh. 5.6 - Prob. 57PPCh. 5.6 - Indicate the number of valence electrons in each...Ch. 5.6 - Prob. 59PPCh. 5.6 - Prob. 60PPCh. 5.6 - Prob. 61PPCh. 5.6 - Prob. 62PPCh. 5.6 - Prob. 63PPCh. 5.6 - Select the element in each pair with the higher...Ch. 5.6 - Prob. 65PPCh. 5.6 - Prob. 66PPCh. 5.6 - Prob. 67PPCh. 5.6 - Prob. 68PPCh. 5.6 - Prob. 69PPCh. 5.6 - Prob. 70PPCh. 5.6 - Prob. 71PPCh. 5.6 - Prob. 72PPCh. 5.6 - Which statements completed with a to e will be...Ch. 5.6 - Which statements completed with a to e will be...Ch. 5.6 - Prob. 75PPCh. 5.6 - a. What is the atomic number of Te? b. How many...Ch. 5 - The chapter sections to review are shown in...Ch. 5 - Prob. 78UTCCh. 5 - Prob. 79UTCCh. 5 - Prob. 80UTCCh. 5 - The chapter sections to review are shown in...Ch. 5 - The chapter sections to review are shown in...Ch. 5 - The chapter sections to review are shown in...Ch. 5 - Prob. 84UTCCh. 5 - Prob. 85APPCh. 5 - Prob. 86APPCh. 5 - Prob. 87APPCh. 5 - Prob. 88APPCh. 5 - Prob. 89APPCh. 5 - Prob. 90APPCh. 5 - Prob. 91APPCh. 5 - Prob. 92APPCh. 5 - a. How many 3d electrons are in Fe? (5.4) b. How...Ch. 5 - a. How many 4d electrons are in Cd? (5.4) b. How...Ch. 5 - Write the abbreviated electron configuration and...Ch. 5 - Prob. 96APPCh. 5 - What do the elements Ca, Sr, and Ba have in common...Ch. 5 - Prob. 98APPCh. 5 - Prob. 99APPCh. 5 - Name the element that corresponds to each of the...Ch. 5 - Prob. 101APPCh. 5 - Prob. 102APPCh. 5 - Select the more metallic element in each pair....Ch. 5 - Select the more metallic element in each pair....Ch. 5 - Of the elements Na, P, CI, and F, which (5.6) a....Ch. 5 - Of the elements K, Ca, Br, and Kr, which (5.6) a....Ch. 5 - Prob. 107APPCh. 5 - Prob. 108APPCh. 5 - Prob. 109CPCh. 5 - Prob. 110CPCh. 5 - Prob. 111CPCh. 5 - Prob. 112CPCh. 5 - Prob. 113CPCh. 5 - The following problems are related to the topics...Ch. 5 - The following problems are related to the topics...Ch. 5 - The following problems are related to the topics...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explain why you could or could not measure the wavelength of a golf ball in flight.arrow_forwardOne type of solar radiation in the upper atmosphere has a frequency of 7.898 1014 Hz; another type has a frequency of 1.20 1015 Hz. (a) In what region of the electromagnetic spectrum does this solar radiation occur? (b) Which of the two types of radiation has the shorter wavelength? Explain your answer.arrow_forwarddescribe waves in terms of frequency, wavelength, and amplitude.arrow_forward
Recommended textbooks for you
 Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Quantum Mechanics - Part 1: Crash Course Physics #43; Author: CrashCourse;https://www.youtube.com/watch?v=7kb1VT0J3DE;License: Standard YouTube License, CC-BY