
Concept explainers
(a)
Interpretation:
Structural formula for methyl thiopropanoate has to be drawn.
Concept Introduction:
General structure of thioester can be represented as shown below,
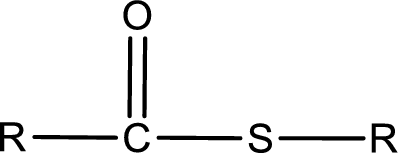
From an IUPAC name, the structure of the thioester can be derived. IUPAC name of thioester consists of two parts. In an IUPAC name of thioester, the first part of the name is the alkyl part and the second part is the acid part. Alkyl group have come from the thiol and acyl part from
The same rule applies for deriving a structure from common name. The only difference is the acyl part name. The acyl part is named using the common name of thiocarboxylic acid.
(b)
Interpretation:
Structural formula for methyl thiopropionate has to be drawn.
Concept Introduction:
General structure of thioester can be represented as shown below,
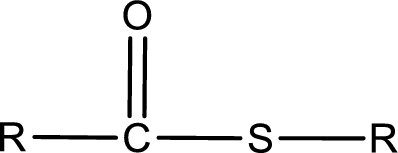
From an IUPAC name, the structure of the thioester can be derived. IUPAC name of thioester consists of two parts. In an IUPAC name of thioester, the first part of the name is the alkyl part and the second part is the acid part. Alkyl group have come from the thiol and acyl part from carboxylic acid.
The same rule applies for deriving a structure from common name. The only difference is the acyl part name. The acyl part is named using the common name of thiocarboxylic acid.
(c)
Interpretation:
Structural formula for ethyl thioacetate has to be drawn.
Concept Introduction:
General structure of thioester can be represented as shown below,
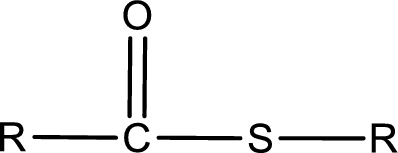
From an IUPAC name, the structure of the thioester can be derived. IUPAC name of thioester consists of two parts. In an IUPAC name of thioester, the first part of the name is the alkyl part and the second part is the acid part. Alkyl group have come from the thiol and acyl part from carboxylic acid.
The same rule applies for deriving a structure from common name. The only difference is the acyl part name. The acyl part is named using the common name of thiocarboxylic acid.
(d)
Interpretation:
Structural formula for ethyl thioethanoate has to be drawn.
Concept Introduction:
General structure of thioester can be represented as shown below,
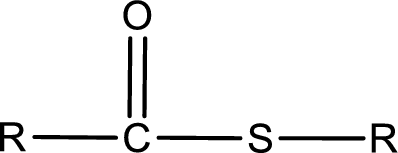
From an IUPAC name, the structure of the thioester can be derived. IUPAC name of thioester consists of two parts. In an IUPAC name of thioester, the first part of the name is the alkyl part and the second part is the acid part. Alkyl group have come from the thiol and acyl part from carboxylic acid.
The same rule applies for deriving a structure from common name. The only difference is the acyl part name. The acyl part is named using the common name of thiocarboxylic acid.
Trending nowThis is a popular solution!

Chapter 5 Solutions
Organic And Biological Chemistry
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardThe Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forward
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardOnly 100% sure experts solve it correct complete solutions need to get full marks it's my quiz okkkk.take your time but solve full accurate okkk chemistry expert solve itarrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning




