
(a)
Interpretation:
Among the given designations, the one or more which may be correct for the given situation has to be chosen.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom.
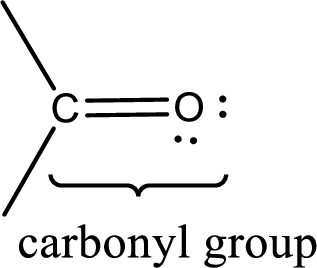
If a hydroxyl group is attached to a carbonyl group means it is known as carboxyl group. This can be represented as shown below,
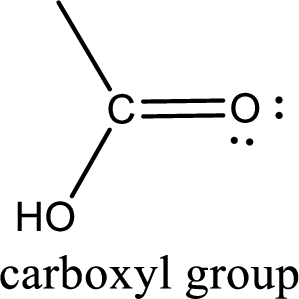
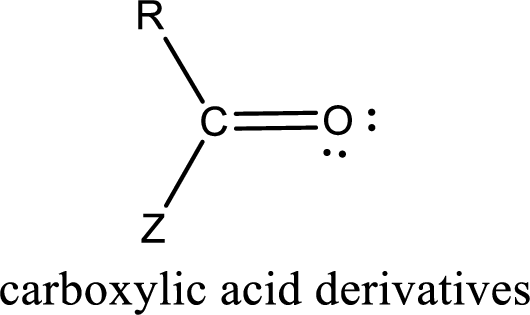
Carbon bonded to an oxygen atom through double bond is known as carbonyl group. The carbonyl group bonded to a R group means it is known as acyl group. If the acyl group is bonded to oxygen, nitrogen or halogen atom then the compound is known as acyl compound. If the acyl group is bonded to a carbon or hydrogen atom, then the compound is known as carbonyl compound. If the carbonyl carbon atom is bonded to a hydroxyl group then it is a carboxylic acid.
(b)
Interpretation:
Among the given designations, the one or more which may be correct for the given situation has to be chosen.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
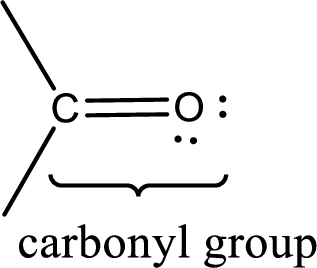
If a hydroxyl group is attached to a carbonyl group means it is known as carboxyl group. This can be represented as shown below,
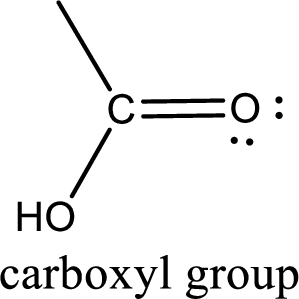
Carboxylic acid derivatives are the ones that are synthesized from or converted to a carboxylic acid. The generalized structural representation of carboxylic acid derivatives is shown below,
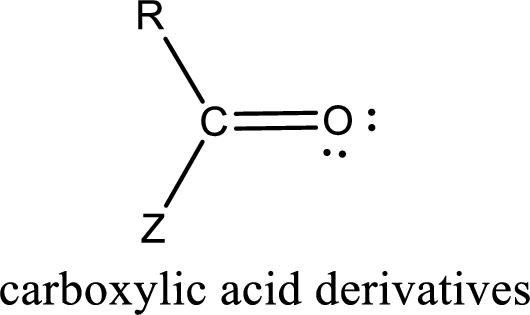
Carbon bonded to an oxygen atom through double bond is known as carbonyl group. The carbonyl group bonded to a R group means it is known as acyl group. If the acyl group is bonded to oxygen, nitrogen or halogen atom then the compound is known as acyl compound. If the acyl group is bonded to a carbon or hydrogen atom, then the compound is known as carbonyl compound. If the carbonyl carbon atom is bonded to a hydroxyl group then it is a carboxylic acid.
(c)
Interpretation:
Among the given designations, the one or more which may be correct for the given situation has to be chosen.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
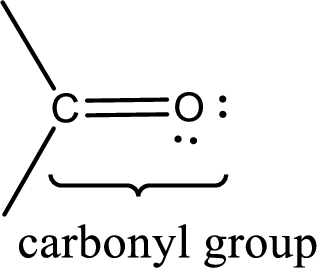
If a hydroxyl group is attached to a carbonyl group means it is known as carboxyl group. This can be represented as shown below,
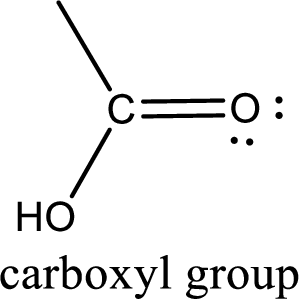
Carboxylic acid derivatives are the ones that are synthesized from or converted to a carboxylic acid. The generalized structural representation of carboxylic acid derivatives is shown below,
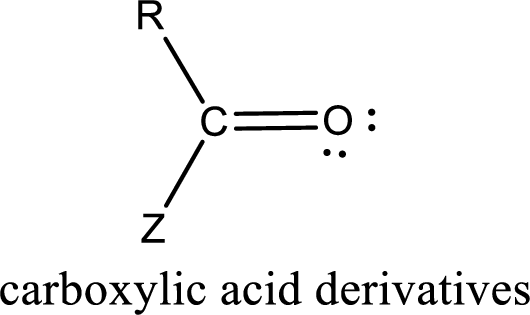
Carbon bonded to an oxygen atom through double bond is known as carbonyl group. The carbonyl group bonded to a R group means it is known as acyl group. If the acyl group is bonded to oxygen, nitrogen or halogen atom then the compound is known as acyl compound. If the acyl group is bonded to a carbon or hydrogen atom, then the compound is known as carbonyl compound. If the carbonyl carbon atom is bonded to a hydroxyl group then it is a carboxylic acid.
(d)
Interpretation:
Among the given designations, the one or more which may be correct for the given situation has to be chosen.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
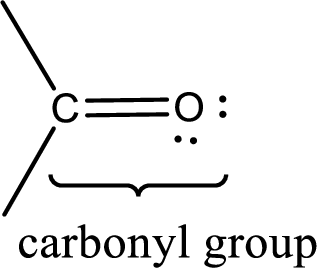
If a hydroxyl group is attached to a carbonyl group means it is known as carboxyl group. This can be represented as shown below,
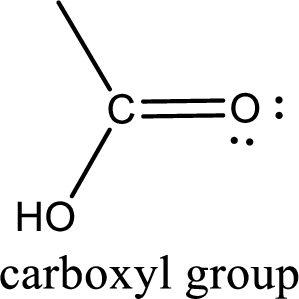
Carboxylic acid derivatives are the ones that are synthesized from or converted to a carboxylic acid. The generalized structural representation of carboxylic acid derivatives is shown below,
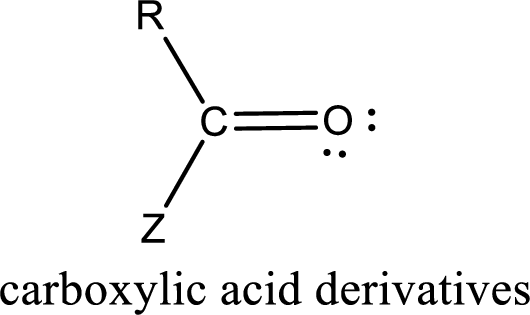
Carbon bonded to an oxygen atom through double bond is known as carbonyl group. The carbonyl group bonded to a R group means it is known as acyl group. If the acyl group is bonded to oxygen, nitrogen or halogen atom then the compound is known as acyl compound. If the acyl group is bonded to a carbon or hydrogen atom, then the compound is known as carbonyl compound. If the carbonyl carbon atom is bonded to a hydroxyl group then it is a carboxylic acid.
Trending nowThis is a popular solution!

Chapter 5 Solutions
Organic And Biological Chemistry
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardThe Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forward
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardOnly 100% sure experts solve it correct complete solutions need to get full marks it's my quiz okkkk.take your time but solve full accurate okkk chemistry expert solve itarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forward

 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





