
Interpretation:
The number of molecules of adenine in one molecule of Sample II needs to be determined.
Concept introduction:
DNA stands for deoxyribonucleic acid and RNA stands for ribonucleic acid. DNA includes four bases such as adenine (A), guanine (G), cytosine (C), and thymine (T). RNA consists of four bases: adenine, cytosine, uracil, and guanine.
Answer to Problem 7STP
Option C is the correct option.
Explanation of Solution
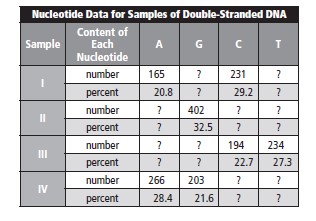
We know that 32.5% of bases is equal to 402. So, one can calculate the total number of bases by using the following formula,
Hence, the total number of bases is
If C = G, then percentage is equal to 32.5% and total percentage after adding is 65%. The percentage left over is 32.5% composed of A and T. By this it can be concluded that 17.5% is A and 17.5% is T.
The number of molecules of adenine is determined as follows,
216 molecules of adenine are there in one molecule of Sample II.
Chapter 23 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Organic Chemistry
Introductory Chemistry (5th Edition) (Standalone Book)
Chemistry: The Central Science (14th Edition)
Chemistry: A Molecular Approach (4th Edition)
Chemistry: The Central Science (13th Edition)
General, Organic, and Biological Chemistry (3rd Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





