
Interpretation:
The name of two types of
Concept introduction:
Proteins are
They polymerise by peptide linkage to form dipeptide, oligopeptide and polypeptide molecules.
Answer to Problem 34A
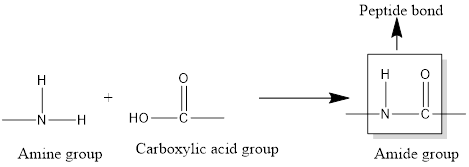
Two types of functional groups that react with each to form peptide bond is amino group and carboxyl group.
The functional group in the peptide bond is amide group.
Explanation of Solution
Peptide bonds are identified by the bonding present between carboxyl group of one amino acid and one amino group of other amino acid. Each peptide bond formation is a condensation reaction that occurs with the elimination of water molecule.
Thus, two functional groups are
When amine group and carboxylic acid reacts with each other, amide group (peptide bond) is formed with the removal of water molecule.
The general
Chapter 23 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Chemistry: The Central Science (13th Edition)
Chemistry: The Central Science (14th Edition)
Organic Chemistry
CHEMISTRY-TEXT
General Chemistry: Principles and Modern Applications (11th Edition)
Essential Organic Chemistry (3rd Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





