
Concept explainers
Interpretation:
The element having ground-state electronic configuration, 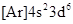 needs to be determined.
needs to be determined.
Concept introduction:
The electrons are arranged around the nucleus of an atom in an increasing order of energy levels and this description of atomic orbitals of atom occupied by electrons is known as electronic configuration.
Answer to Problem 95A
The element having ground-state electronic configuration, 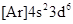 is iron,
is iron, 
Explanation of Solution
Electrons are distributed in the orbitals the of subshell. The specific region of space in which the movement of electrons are confined is said to be shells which are divided into subshells and are s-, p-, d-, and f-. Among these subshells, the electrons are grouped as orbitals.
The number of electrons that these subshells can hold are:
s-block - 2
p-block - 6
d-block - 10
f-block - 14
The increasing order of energy of shells, subshell is:
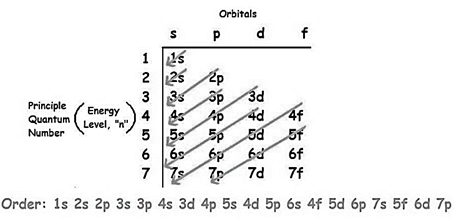
As the number of electrons for a neutral atom is equal to the 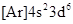 will be:
will be:
The atomic number of 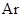 is 18 and 8 electrons in
is 18 and 8 electrons in 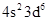 so, the total number of electrons is
so, the total number of electrons is 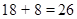 Thus, the element having atomic number 26 is represented by the ground-state electronic configuration,
Thus, the element having atomic number 26 is represented by the ground-state electronic configuration, 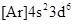 The element having atomic number 26 is iron,
The element having atomic number 26 is iron, 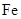
Chapter 21 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Chemistry: Structure and Properties (2nd Edition)
Chemistry: The Central Science (14th Edition)
Chemistry: A Molecular Approach
Chemistry: A Molecular Approach (4th Edition)
Organic Chemistry (8th Edition)
Organic Chemistry (9th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





