
Interpretation:
The structural formula of heptane needs to be drawn.
Concept introduction:
The hydrocarbon compounds that contains single bond(s) between carbon atom(s) are said to be saturated hydrocarbon. Hydrocarbon compounds containing single bonds only are said to be
Answer to Problem 56A
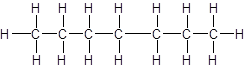
Explanation of Solution
Alkanes are compounds containing carbon and hydrogen atoms having single bond(s) between carbon atom(s) in the structure. For number of carbons atoms in alkane chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
The given name is heptane that means the parent chain has 7 number of carbons in the chain and there is no substituent in the chain. So, the structural formula of heptane is:
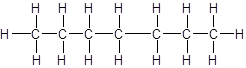
Interpretation:
The structural formula of 2-methylhexane needs to be drawn.
Concept introduction:
The hydrocarbon compounds that contains single bond(s) between carbon atom(s) are said to be saturated hydrocarbon. Hydrocarbon compounds containing single bonds only are said to be alkane.
Answer to Problem 56A
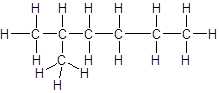
Explanation of Solution
The given name is 2-methylhexane that means the parent chain has 6 number of carbons in straight chain and a methyl substituent at position 2 in the chain. So, the structural formula of 2-methylhexane is:
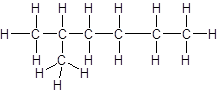
Interpretation:
The structural formula of 2, 3-dimethylpentane needs to be drawn.
Concept introduction:
The hydrocarbon compounds that contains single bond(s) between carbon atom(s) are said to be saturated hydrocarbon. Hydrocarbon compounds containing single bonds only are said to be alkane.
Answer to Problem 56A
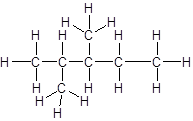
Explanation of Solution
The given name is 2, 3-dimethylpentane that means the parent chain has 5 number of carbons in straight chain and two methyl substituents at position 2 and 3 in the chain. So, the structural formula of 2, 3-dimethylpentane is:
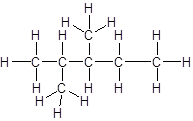
Interpretation:
The structural formula of 2, 2-dimethylpropane needs to be drawn.
Concept introduction:
The hydrocarbon compounds that contains single bond(s) between carbon atom(s) are said to be saturated hydrocarbon. Hydrocarbon compounds containing single bonds only are said to be alkane.
Answer to Problem 56A
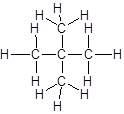
Explanation of Solution
The given name is 2, 2-dimethylpropane that means the parent chain has 3 number of carbons in straight chain and two methyl substituents at position 2 in the chain. So, the structural formula of 2, 2-dimethylpropane is:
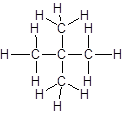
Chapter 21 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Chemistry: A Molecular Approach
Chemistry: Structure and Properties (2nd Edition)
Chemistry: The Central Science (14th Edition)
Chemistry: Structure and Properties
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





