
Interpretation:
The formula of the compound should be determined on the basis of following figure.
A.
B.
C.
D.
E.
Concept introduction:
Number of moles is equal to the ratio of given mass to the molar mass.
The mathematical expression is given by:
Number of moles =
Molar mass of the molecule is equal to the sum of the masses of atoms present in the molecule.
Answer to Problem 16STP
Option (D) is correct that is
Explanation of Solution
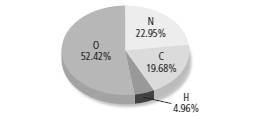
According to the given figure,
Percentage of carbon = 19.68 %
Percentage of oxygen = 52.42 %
Percentage of hydrogen = 4.96 %
Percentage of nitrogen = 22.95 %
Let, total mass of the compound = 100 grams.
Thus, mass of carbon (
Mass of oxygen (
Mass of hydrogen (
Mass of nitrogen (
Now,
Molar mass of carbon = 12.011 g/mole
Number of moles of carbon =
=
Molar mass of oxygen = 15.999 g/mole
Number of moles of oxygen =
=
Molar mass of hydrogen = 1.008 g/mole
Number of moles of hydrogen =
=
Molar mass of nitrogen = 14.007 g/mole
Number of moles of nitrogen =
=
Divide each number of moles by smallest number 1.64.
Number of moles of carbon =
= 1 mole
Number of moles of oxygen =
= 2 moles
Number of moles of hydrogen =
= 3 moles
Number of moles of nitrogen =
= 1 mole
Thus, the formula of compound is
Hence, option (D) is correct.
Chapter 21 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Organic Chemistry (9th Edition)
Introductory Chemistry (5th Edition) (Standalone Book)
General, Organic, and Biological Chemistry (3rd Edition)
Chemistry: Structure and Properties (2nd Edition)
Introductory Chemistry (6th Edition)
Chemistry: Structure and Properties
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





