
Concept explainers
a.
Interpretation:
The name of the following structural formula needs to be determined:
Concept introduction:
The hydrocarbon compounds that contains single bond(s) between carbon atom(s) are said to be saturated hydrocarbon. Hydrocarbon compounds containing single bonds only are said to be
a.
Answer to Problem 55A
Pentane.
Explanation of Solution
In order to give the name to the alkane following steps are followed:
1. The parent (longest) continuous carbon chain is identified.
2. The ending of the parent chain for alkane (-e) is -ane.
3. Name should be written in alphabetical order and numbering should be done in such a way that the substituent group gets lowest number.
4. Hyphen is used to connect the number to the name.
For number of carbons atoms in alkane chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
The given structure is:
The parent chain contains 5 carbon as shown:
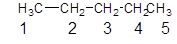
Since, the parent chain contains 5 carbon atoms and only single bonds are present so, the name of the compound is pentane.
b.
Interpretation:
The name of the following compound should be determined:
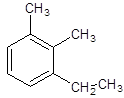
Concept introduction:
The ring structures of the compound having uncommon stability due to delocalized pi electron density shared in between all the carbon atoms of the ring is said to be an aromatic compound.
b.
Answer to Problem 55A
1-ethyl-2, 3-dimethylbenzene.
Explanation of Solution
In order to give the name to the multiple substituted
- For single substituted aromatic compound (when the substituent contains six or fewer carbons), the name of the substituted group is written first followed by the name of the aromatic compound.
- For single substituted aromatic compound (when the substituent contains more than six carbons), the name of the aromatic compound is written first followed by the name of the substituted group.
- For single substituted aromatic compound, the numbering on the ring is done in such a way that the multiple substituents get the lowest number.
The given structure is:
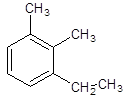
The given structure is trisubstituted substituted aromatic compound, benzene that contains two carbon chain, ethyl and 2 substituents as methyl so, numbering is done in a way:
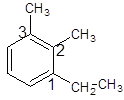
Hence, the name of the compound is 1-ethyl-2, 3-dimethylbenzene.
c.
Interpretation:
The name of the following structural formula needs to be determined:
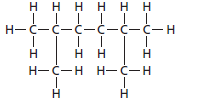
Concept introduction:
The hydrocarbon compounds that contains single bond(s) between carbon atom(s) are said to be saturated hydrocarbon. Hydrocarbon compounds containing single bonds only are said to be alkane.
c.
Answer to Problem 55A
2, 5-dimethylhexane.
Explanation of Solution
The given structure is:
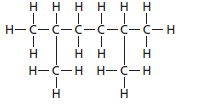
The parent chain contains 6 carbon atoms and 4 substituents so, the numbering is done as shown:
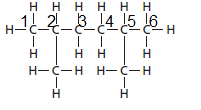
Since, the parent chain contains 6 carbon atoms and 2 methyl substituents at position 2 and 5 so, the name of the compound is 2, 5-dimethylhexane.
d.
Interpretation:
The name of the following structural formula needs to be determined:
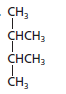
Concept introduction:
The hydrocarbon compounds that contains single bond(s) between carbon atom(s) are said to be saturated hydrocarbon. Hydrocarbon compounds containing single bonds only are said to be alkane.
d.
Answer to Problem 55A
2, 3-dimethylbutane.
Explanation of Solution
The given structure is:
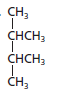
The parent chain contains 4 carbon atoms and 2 substituents so, the numbering is done as shown:
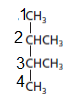
Since, the parent chain contains 4 carbon atoms and 2 methyl substituents at position 2 and 3 so, the name of the compound is 2, 3-dimethylbutane.
Chapter 21 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Chemistry: Structure and Properties
Organic Chemistry (9th Edition)
Introductory Chemistry (6th Edition)
Organic Chemistry
CHEMISTRY-TEXT
Chemistry: The Central Science (14th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





