
Concept explainers
Interpretation:
The name of compound should be determined for the following skeletal structure.
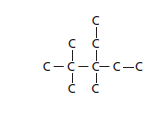
- 2, 2, 3-trimethyl-3-ethylpentane
- 3-ethyl-3, 4, 4-trimethylpentane
- 2-butyl-2-ethylbutane
- is 3-ethyl-2, 2, 3-trimethylpentane
- 2, 2-dimethyl, 3-diethyl, 3-methylpropane
Concept introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming
- First choose the longest continuous chain of carbon atoms known as parent chain and determines the base name of alkane.
- The numbering of parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
Answer to Problem 15STP
Option (D) is correct that is 3-ethyl-2, 2, 3-trimethylpentane.
Explanation of Solution
The skeletal structure of compound:
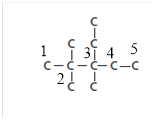
- The longest continuous chain contains five carbon atoms and the base name is pentane.
- Two substituents that are two methyl groups are present on second carbon atom of the chain.
- Two substituents that are ethyl and methyl group are present on third carbon atom of the chain.
- The numbering for the substituents is given as: 2, 2, 3-trimethyl and 3-ethyl
- Since, ethyl is starting with alphabet “e” thus, comes first in the naming of the compound.
Therefore, the name of the compound is 3-ethyl-2, 2, 3-trimethylpentane.
Hence, option (D) is correct.
Chapter 21 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Chemistry: The Central Science (13th Edition)
Chemistry: Structure and Properties
Introductory Chemistry (6th Edition)
Chemistry: The Central Science (14th Edition)
General, Organic, and Biological Chemistry (3rd Edition)
General Chemistry: Principles and Modern Applications (11th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





