
(a)
Interpretation: The IUPAC name of the following compound should be determined:
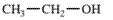
Concept Introduction:
In order to give the IUPAC name to the alcohol following steps are followed:
1. The parent (longest)
2. The ending of the parent chain from alkane (-e) is changed to -ol and the number is used to locate the -OH group of alcohol.
3. Name should be written in alphabetical order and numbering should be done in such a way that hydroxy group gets lowest number.
4. Configuration should be specified is there exist any cis-trans isomerism.
For number of carbons atoms in chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
(b)
Interpretation: The IUPAC name of the following compound should be determined:
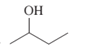
Concept Introduction:
In order to give the IUPAC name to the alcohol following steps are followed:
1. The parent (longest) alkane chain is identified.
2. The ending of the parent chain from alkane (-e) is changed to -ol and the number is used to locate the -OH group of alcohol.
3. Name should be written in alphabetical order and numbering should be done in such a way that hydroxy group gets lowest number.
4. Configuration should be specified is there exist any cis-trans isomerism.
For number of carbons atoms in chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
(c)
Interpretation: The IUPAC name of the following compound should be determined:
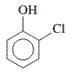
Concept Introduction:
In order to give the IUPAC name to the alcohol following steps are followed:
1. The parent (longest) alkane chain is identified.
2. The ending of the parent chain from alkane (-e) is changed to -ol and the number is used to locate the -OH group of alcohol.
3. Name should be written in alphabetical order and numbering should be done in such a way that hydroxy group gets lowest number.
4. Configuration should be specified is there exist any cis-trans isomerism.
For number of carbons atoms in chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
Basic Chemistry
- Phenol is Select one: a. CH3CH2CH2OH b. C8H11OH c. CH3CH2CH2CH2CH2OH d. C6H5OHarrow_forwardGive the IUPAC name for the following compound: re OH Select one: O A. 5-ethyl-2-methyl-3-heptanol O B. 3-methyl-1-(3-pentyl)-2-butanol O C. 3-ethyl-1-isopropyl-1-pentanol O D. 3-ethyl-6-methyl-5-heptanolarrow_forwardWhat is the IUPAC name of the compound below? H. O 3.7-dimethyl-6-one-4-octynal O 2.6-dimethyl-3-one-4-octynal 3,7-dimethyl-6-oxo-4-octynal 2,6-dimethyl-3-oxo-4-octynalarrow_forward
- What is the IUPAC name of the molecule below? CH3 CH;CH,CHCH CHCH CH C-OH Select one: a. octanoic acid O b. 6-ethyl-4-methylhexanoic acid O c. 4,6-dimethyloctanoic acid O d. 3,5-dimethyloctanoic acidarrow_forwardWhich of the following compounds is most soluble in water? a) CH3-CH2-CH2-CH2-COOH b) CH3-CH2-CH2-CH2-OH c)CH3-COOH d)CH3-CH2-CH3 e) CH3-CH2-CH2-O-CH3arrow_forwardFollowing are IUPAC names and structural formulas for compound Divide each name into a prefix, an infix, and a suffix and specify the information about the structural formula that is contained in each part of the name.arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning





