
Concept explainers
(a)
Interpretation: The IUPAC name of the following compound should be determined:
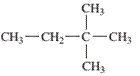
Concept Introduction:
In order to give the IUPAC name to the
1. The parent (longest) continuous carbon chain is identified.
2. The ending of the parent chain for alkane (-e) is -ane.
3. Name should be written in alphabetical order and numbering should be done in such a way that the substituent group gets lowest number.
4. Hyphen is used to connect the number to the name.
For number of carbons atoms in alkane chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
(b)
Interpretation: The IUPAC name of the following compound should be determined:
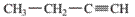
Concept Introduction:
The hydrocarbon compounds containing only hydrogen and carbon atoms that contain multiple bond(s) are said to be
In order to give the name to the unsaturated hydrocarbon following steps are followed:
1. The parent (longest) continuous carbon chain containing multiple bonds between the carbon atoms is selected.
2. While writing the name of alkyne, the suffix “ane” of the corresponding alkane is replaced by “yne”.
3. Name should be written in alphabetical order and numbering should be done in such a way that the multiple bond and substituent group gets lowest number.
4. Hyphen is used to connect the number to the name.
For number of carbons atoms in alkane chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
(c)
Interpretation: The IUPAC name of the following compound should be determined:

Concept Introduction:
The hydrocarbon compounds containing only hydrogen and carbon atoms that contain multiple bond(s) are said to be unsaturated hydrocarbon. Compounds containing double bonds are said to be alkene whereas compounds containing triple bonds are said to be alkyne.
In order to give the name to the unsaturated hydrocarbon following steps are followed:
1. The parent (longest) continuous carbon chain containing multiple bonds between the carbon atoms is selected.
2. While writing the name of alkene, the suffix “ane” of the corresponding alkane is replaced by “ene”.
3. Name should be written in alphabetical order and numbering should be done in such a way that the multiple bond and substituent group gets lowest number.
4. Hyphen is used to connect the number to the name.
For number of carbons atoms in alkane chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
Basic Chemistry
- 12.38 The compound frambinone has the taste of raspberries and has been used in weight loss. Identify the functional groups in frambinone. (12.1, 12.3) HO O CH,—CH,—C–CH3 14 Frambinone odioarrow_forward12.52 Draw the condensed structural or line-angle formula for the alkene, aldehyde, or ketone product of each of the following reactions: (12.4) H, heat a. ОН CH; OH b. CHз — СH — СH-CH, OH [0] ОН d. CH3-CH2-CH2-CH-CH3arrow_forward10. Draw the structural formula for the product in each of the following addition reactions for an alkene: (11.7) a) CH3-CH2-C=CH-CH3 + H,O CH3-CH₂ b) CH 3 CH3-CH-C=CH-CH3 + H₂ CH3 + H₂ Pt H Niarrow_forward
- 12.42 Which of the following will give a positive Tollens' test? (12.4) 1-propanol b. 2-propanol c. hexanal a.arrow_forward12.58 Draw the condensed structural or line-angle formula, if cyclic, for each of the following: (12.3) a. formaldehyde c. 3-methyl-2-hexanone d. 3,5-dimethylhexanal b. 2-chlorobutanalarrow_forward(12.3)Which of the following has the strongest dispersion force between its molecules? O CH3CH3 O CH3CH₂CH₂CH₂CH3 O All of these have the dispersion forces with the same strength. O CH3CH₂CH₂CH3 O CH3CH₂CH₂CH₂CH₂CH3arrow_forward
- Rank the following substances in order of increasing acidity: Which, if any, of the four compounds is a strong enough acid to react almost completely OH CHyCH3 CHyČCH,ČCH, CH,COH Acetone Pentane-2,4-dione Phenol Acetic acid with NaOH? (pK, 19.3) (pK, = 9) (pK, = 9.9) (pK,4.76) (The pK, of H20 is 15.74.)arrow_forwardWhat is the IUPAC name of the following compounds?arrow_forwardQ117arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





