
Concept explainers
(a)
Interpretation: The IUPAC name of the following compound should be determined:
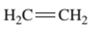
Concept Introduction:
The hydrocarbon compounds are the compound containing only hydrogen and carbon atoms, compounds that contain carbon-carbon multiple bond(s) are said to be
In order to give the name to the unsaturated hydrocarbon following steps are followed:
1. The parent (longest) continuous carbon chain containing multiple bonds between the carbon atoms is selected.
2. While writing the name of alkene, the suffix “ane” of the corresponding
3. Name should be written in alphabetical order and numbering should be done in such a way that the multiple bond and substituent group gets lowest number.
4. Hyphen is used to connect the number to the name.
For number of carbons atoms in alkane chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
(b)
Interpretation: The IUPAC name of the following compound should be determined:
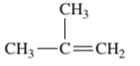
Concept Introduction:
The hydrocarbon compounds are the compound containing only hydrogen and carbon atoms, compounds that contain carbon-carbon multiple bond(s) are said to be unsaturated hydrocarbon. Compounds containing double bonds are said to be alkene whereas compounds containing triple bonds are said to be alkyne.
In order to give the name to the unsaturated hydrocarbon following steps are followed:
1. The parent (longest) continuous carbon chain containing multiple bonds between the carbon atoms is selected.
2. While writing the name of alkene, the suffix “ane” of the corresponding alkane is replaced by “ene”.
3. Name should be written in alphabetical order and numbering should be done in such a way that the multiple bond and substituent group gets lowest number.
4. Hyphen is used to connect the number to the name.
For number of carbons atoms in alkane chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
(c)
Interpretation: The IUPAC name of the following compound should be determined:
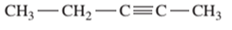
Concept Introduction:
The hydrocarbon compounds are the compound containing only hydrogen and carbon atoms, compounds that contain carbon-carbon multiple bond(s) are said to be unsaturated hydrocarbon. Compounds containing double bonds are said to be alkene whereas compounds containing triple bonds are said to be alkyne.
In order to give the name to the unsaturated hydrocarbon following steps are followed:
1. The parent (longest) continuous carbon chain containing multiple bonds between the carbon atoms is selected.
2. While writing the name of alkyne, the suffix “ane” of the corresponding alkane is replaced by “yne”.
3. Name should be written in alphabetical order and numbering should be done in such a way that the multiple bond and substituent group gets lowest number.
4. Hyphen is used to connect the number to the name.
For number of carbons atoms in alkane chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
Basic Chemistry
- 3. What is the IUPAC name for a,b.c and the common name of c? CH2-CH3 ÇH3 CH3-CH2-CH-CH2-CH-CH3 CH3-CH2-C-CH3 Br CH3 a. b. CH3-CH,-CI с.arrow_forwardWhat is the IUPAC name of the compound below? H. O 3.7-dimethyl-6-one-4-octynal O 2.6-dimethyl-3-one-4-octynal 3,7-dimethyl-6-oxo-4-octynal 2,6-dimethyl-3-oxo-4-octynalarrow_forward3. Write the common name for: HC=CH, 4. Write the IUPAC name and the common name for: H3C- CH CH2 -CH2 -CH3 CH3 IUPAC toarrow_forward
- Which one of the following compounds is an alkene Select one: O a. H-CEC. H0-C-C-CH, O c. H,C-C=C-CH, Od. H. H-C=C-C-C-H H.arrow_forwardGive the IUPAC names for the following compounds. 1. 2. H3C-C(CH3)2CH=C-CH3 H Br 3. OH Br 4. CI CIarrow_forward4. Use the IUPAC Nomenclature System to name each of the following compounds: a. b. CH,CCH₂CH, 0 HCCHCH₂CH₂ CI O -C-C-CH₂ Br CHỊCH,CH,CH, CH,CCH₂CH₂CH CH, CI CH₂CHCH₂CH Br CH, Ô CH,CCH.CCH.CH.CH, OH 0 CH₂ CH,CCH₂CH.CH,CHCH,C-H H,CHCH.CH CH₂arrow_forward
- What is the IUPAC name of the following compound? CH3 CH3-C=C-CH,-CH-CH3 Select one: A. 5-methyl-3-hexyne B. 2-methyl-4-hexyne C. 6-methyl-3-hexyne D. 5-methyl-2-hexyne E. 4-methyl-2-hexynearrow_forwardGive the common name and IUPAC name: 4. (CH3)3CCH2COBr 5. C3H7CONHCH3 6. C-C-C-C-C-C-COCI C-C-C C | Carrow_forwardWhat are the systematic (IUPAC) names for the compounds shown? A. OH H3C systematic (IUPAC) name: B. H3C-CH₂-CH-CH₂ LL CH3 OH systematic (IUPAC) name:arrow_forward
- 2. Name the following Alkanes using the IUPAC nomenclature rules: H HHH H-C-C-C-C-H H HHH a. H- -HH H. H. H- H. H. H-C H b. C.arrow_forwardGive IUPAC name for the following compounds (A and B): A CH3 Br. H3C- CH3 CH3 H3C CI CH3arrow_forward13.34 Name each of the following using the IUPAC Nomenclature System: ÇI O b. a. Cl-C-C-CH, CIarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
