
(a)
Interpretation: The IUPAC name of the following compound should be determined:
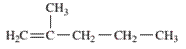
Concept Introduction:
The hydrocarbon compounds containing only hydrogen and carbon atoms that contain multiple bond(s) are said to be
In order to give the name to the unsaturated hydrocarbon following steps are followed:
1. The parent (longest) continuous carbon chain containing multiple bonds between the carbon atoms is selected.
2. While writing the name of alkene, the suffix “ane” of the corresponding
3. Name should be written in alphabetical order and numbering should be done in such a way that the multiple bond and substituent group gets lowest number.
4. Hyphen is used to connect the number to the name.
For number of carbons atoms in alkane chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
(b)
Interpretation: The IUPAC name of the following compound should be determined:

Concept Introduction:
In order to give the IUPAC name to the alkane following steps are followed:
1. The parent (longest) continuous carbon chain is identified.
2. The ending of the parent chain for alkane (-e) is -ane.
3. Name should be written in alphabetical order and numbering should be done in such a way that the substituent group gets lowest number.
4. Hyphen is used to connect the number to the name.
For number of carbons atoms in alkane chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
(c)
Interpretation: The IUPAC name of the following compound should be determined:
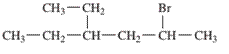
Concept Introduction:
In order to give the IUPAC name to the alkane following steps are followed:
1. The parent (longest) continuous carbon chain is identified.
2. The ending of the parent chain for alkane (-e) is -ane.
3. Name should be written in alphabetical order and numbering should be done in such a way that the substituent group gets lowest number.
4. Hyphen is used to connect the number to the name.
For number of carbons atoms in alkane chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
Basic Chemistry
- Q117arrow_forward10. Draw the structural formula for the product in each of the following addition reactions for an alkene: (11.7) a) CH3-CH2-C=CH-CH3 + H,O CH3-CH₂ b) CH 3 CH3-CH-C=CH-CH3 + H₂ CH3 + H₂ Pt H Niarrow_forwardComplete the following reactions. Please answer I really need helparrow_forward
- 11:17 Worksheet 3 Question 2 of 10 nieaf tis Cnty 2. [Short answer] What is the IUPACname for the compound shown below? Short Answer Type Answer Here 140 Next Question Previous Questionarrow_forwardAnswer the last two items onlyarrow_forwardKindly give the IUPAC names of the following alcohol compounds: 1. 2. 3. 4. 5. OH (CH₂)₂CHCH₂CH(CH₂CH₂CH(OH)CH₂CH₂ H₂CO LOCH, SHarrow_forward
- S 4 Draw all possible isomers for the chemical formula C₂H₂O. Select 2 000 000 → % 5 C Draw H 6 O Rings MacBook Pro & 7 * 8 More ( 9 ) 0arrow_forward6. (4 pts) Which one of these is most likely to smell bad? ) Amines with more than six carb b) aqueous HCI c) aqued a) water a) butyl amine c) N-butylbutanamide b) butyl butanoate d) sodium butanoate Saponification" may be defined as: pasic hydrolysis of an ester b) basic hydrolysis of an amide c) reduction of an ester d) reduction of an amide Condensation" polymers are called that because: a) they are smaller than other polymers d) they are only made in labs In carhons in the starting materials.arrow_forwardWhat is the correct IUPAC name of the following compound? (5)arrow_forward
- 3. What is the IUPAC name for the following compounds? (2 pts.) a) b) H se H OH O Harrow_forwardWrite the IUPAC name if the following:arrow_forwardHello please help me with my homework, I don't have enough time today is the deadline of my homework but I have another homework to do that's why I am not able to do this. Thank you so much, very much appreciated ??arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



