
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
5th Edition
ISBN: 9780321967466
Author: Karen C. Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 16.1, Problem 16.9QAP
Interpretation Introduction
Interpretation: What will be the oxidation product of given compounds if they are converted to carboxylic acids? Draw the condensed or line angle structural formula of each product obtained.
a)
b) 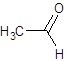
c) 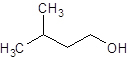
d) 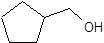
Concept introduction:
(a) Condensed structure represents an organic compound in the form of line of text. However for cyclic compounds we can represent them in condensed structural formula so we draw line angle structural formula for them.
To draw line angle structural formula we should follow the given rules
- In the structure the carbon and hydrogen atoms are not shown, all other atoms are shown.
- Lines are used to show the bonds between carbon and hydrogen and it is assumed that the unfilled valences of carbon are satisfied by hydrogen atoms.
- The end of lines show carbon and the vertices also show the carbon atoms.
(b) Complete oxidation of
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
Ch. 16.1 - What carboxylic acid is responsible for the pain...Ch. 16.1 - What carboxylic acid is found in vinegar?Ch. 16.1 - Prob. 16.3QAPCh. 16.1 - Draw the condensed structural formula and give the...Ch. 16.1 - Prob. 16.5QAPCh. 16.1 - Prob. 16.6QAPCh. 16.1 - Draw the condensed structural formula for each of...Ch. 16.1 - Prob. 16.8QAPCh. 16.1 - Prob. 16.9QAPCh. 16.1 - Prob. 16.10QAP
Ch. 16.2 - Prob. 16.11QAPCh. 16.2 - Prob. 16.12QAPCh. 16.2 - Prob. 16.13QAPCh. 16.2 - Prob. 16.14QAPCh. 16.2 - Prob. 16.15QAPCh. 16.2 - Prob. 16.16QAPCh. 16.2 - Prob. 16.17QAPCh. 16.2 - Prob. 16.18QAPCh. 16.2 - Prob. 16.19QAPCh. 16.2 - Prob. 16.20QAPCh. 16.3 - Prob. 16.21QAPCh. 16.3 - Prob. 16.22QAPCh. 16.3 - Prob. 16.23QAPCh. 16.3 - Prob. 16.24QAPCh. 16.3 - Prob. 16.25QAPCh. 16.3 - Prob. 16.26QAPCh. 16.3 - 16.27 Give the IUPAC and common names,if any, of...Ch. 16.3 - Prob. 16.28QAPCh. 16.4 - Prob. 16.29QAPCh. 16.4 - Prob. 16.30QAPCh. 16.4 - Prob. 16.31QAPCh. 16.4 - Prob. 16.32QAPCh. 16.4 - Prob. 16.33QAPCh. 16.4 - Prob. 16.34QAPCh. 16.5 - Prob. 16.35QAPCh. 16.5 - Prob. 16.36QAPCh. 16.5 - Prob. 16.37QAPCh. 16.5 - Prob. 16.38QAPCh. 16.5 - Prob. 16.39QAPCh. 16.5 - Prob. 16.40QAPCh. 16 - Prob. 16.41UTCCh. 16 - Prob. 16.42UTCCh. 16 - Prob. 16.43UTCCh. 16 - Prob. 16.44UTCCh. 16 - Prob. 16.45UTCCh. 16 - Prob. 16.46UTCCh. 16 - Give the IUPAC and common names, if any, for each...Ch. 16 - Prob. 16.48AQAPCh. 16 - Prob. 16.49AQAPCh. 16 - Prob. 16.50AQAPCh. 16 - Prob. 16.51AQAPCh. 16 - Prob. 16.52AQAPCh. 16 - Prob. 16.53AQAPCh. 16 - Prob. 16.54AQAPCh. 16 - Prob. 16.55AQAPCh. 16 - Prob. 16.56AQAPCh. 16 - Prob. 16.57AQAPCh. 16 - Prob. 16.58AQAPCh. 16 - Prob. 16.59AQAPCh. 16 - Prob. 16.60AQAPCh. 16 - Prob. 16.61AQAPCh. 16 - Prob. 16.62AQAPCh. 16 - Prob. 16.63AQAPCh. 16 - Prob. 16.64AQAPCh. 16 - Prob. 16.65CQCh. 16 - Prob. 16.66CQCh. 16 - Prob. 16.67CQCh. 16 - Prob. 16.68CQCh. 16 - Prob. 16.69CQCh. 16 - Prob. 16.70CQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY