
Concept explainers
Interpretation:
The approximate pH value of five “everyday” solutions needs to be listed.
Concept Introduction:
The acidity or alkalinity of a solution is expressed by determining PH of the solution. PH of a solution is defined as negative logarithm of the concentration of H+ ion in the solution. PH is expressed as-
Answer to Problem 27A
| Sl. No. | Everyday solutions | PH |
| 1. | Ammonia | 12 |
| 2. | Blood | 7-8 |
| 3. | Milk | 6-7 |
| 4. | Vinegar | 2-3 |
| 5. | Lemon | 2 |
In the above table, thepH of the solutions decrease from ammonia to lemon therefore the acidity of the given solutions increase. Ammonia solution is basic whereas the solution of lemon is acidic.
Explanation of Solution
The pH scale is represented as follows:
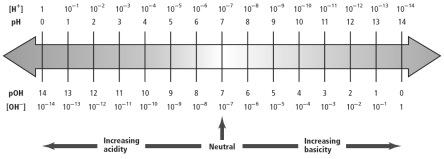
The scale of pH is from 1 to 14. With increasing pH from 1 to 14, the basisity of the solution increases therefore the acidity of the solution decreases and with decreasing pH from 14 to 1,the acidity of the solution increases, therefore, the basicity of the solution decreases. At pH ~ 7, the solution becomes neutral.
| Sl. No. | Everyday solutions | PH |
| 1. | Ammonia | 12 |
| 2. | Blood | 7-8 |
| 3. | Milk | 6-7 |
| 4. | Vinegar | 2-3 |
| 5. | Lemon | 2 |
In the above table thepH of the solutions decrease therefore the acidity of the given solutions increase.Ammonia solution is basic, whereas the solution of lemon is acidic.
Chapter 16 Solutions
World of Chemistry, 3rd edition
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





