
Concept explainers
(a)
Interpretation:
The electronic configuration of
Concept Introduction:
Molecular orbital theory is a method that shows that how atomic orbitals combine with other or with each other to form bonding and antibonding orbitals. It is used to determine the molecular structure of a molecule. The
(a)
Answer to Problem 48E
The electronic configuration of
Explanation of Solution
The given molecule is
The molecular orbital diagram for
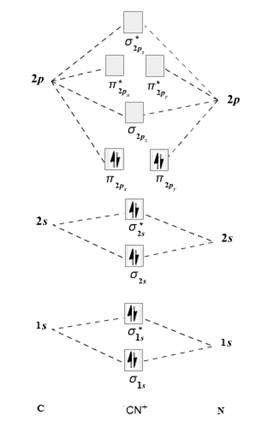
Therefore, the electronic configuration of
The bond order is calculated as follows:
Substitute electrons in antibonding MO and bonding MO for
Therefore, the bond order of
(b)
Interpretation:
The electronic configuration of
Concept Introduction:
Molecular orbital theory is a method that shows that how atomic orbitals combine with other or with each other to form bonding and antibonding orbitals. It is used to determine the molecular structure of a molecule. The atomic number represents the number of electrons in an atom.
(b)
Answer to Problem 48E
The electronic configuration of
Explanation of Solution
The given molecule is
The molecular orbital diagram for
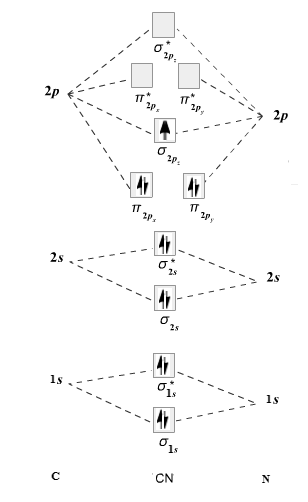
Therefore, the electronic configuration of
The bond order is calculated as follows:
Substitute electrons in antibonding MO and bonding MO for
Therefore, the bond order of
(c)
Interpretation:
The electronic configuration of
Concept Introduction:
Molecular orbital theory is a method that shows that how atomic orbitals combine with other or with each other to form bonding and antibonding orbitals. It is used to determine the molecular structure of a molecule. The atomic number represents the number of electrons in an atom.
(c)
Answer to Problem 48E
The electronic configuration of
The order of increasing bond length is
Explanation of Solution
The given molecule is
The molecular orbital diagram for
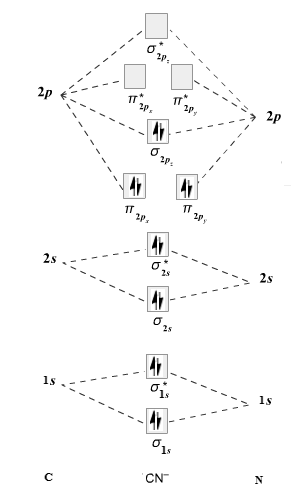
Therefore, the electronic configuration of
The bond order is calculated as follows:
Substitute electrons in antibonding MO and bonding MO for
Therefore, the bond order of
The relationship among bond energy, bond length and bond order is as follows:
The bond order of
The bond order is directly proportional to bond energy. Therefore, the order of increasing bond energy is
Want to see more full solutions like this?
Chapter 14 Solutions
Chemical Principles
- Predict the valence electron molecular orbital configurations for the following, and state whether they will be stable or unstable ions. (a) Na,2+ (b) Mg,2 (c) AI,2 (d) Si,2 (e) p2+ (f) s,2 (g) F,2 (h) Ar,2 40. Predict the valence electron molecular orbital configurations for the following, and state whether they will be stable or unstable ions. (a) Na22+ (b) Mg22+ (c) Al22+ (d) Si22+ (e) P22+ (f) S22+ (g) F22+ (h) Ar22+arrow_forwardAmong the following, which has the shortest bond and which has the longest: Li2, B2, C2, N2, O2?arrow_forwardFClO2 and F3ClO can both gain a fluoride ion to form stable anions. F3ClO and F3ClO2 will both lose a fluoride ion to form stable cations. Draw the Lewis structures and describe the hybrid orbitals used by chlorine in these ions.arrow_forward
- Using the molecular orbital model, write electron configurations for the following diatomic species and calculate the bond orders. Which ones are paramagnetic? Place the species in order of increasing bond length and bond energy. a. CN+ b. CN c. CNarrow_forwardCould the anion Li2 exist? What is the ions bond order?arrow_forwardDescribe the molecular orbital configurations of C2, C2, and C22. What are the bond orders of these species? Arrange the three species by increasing bond length. Arrange the species by increasing bond enthalpy. Explain these arrangements of bond length and bond enthalpy.arrow_forward
- Predict the molecular structure (including bond angles) for each of the following. (See Exercises 115 and 116.) a. XeCl2 b. ICl3 c. TeF4 d. PCl5arrow_forwardUsing the molecular orbital model, write electron configurations for the following diatomic species and calculate the bond orders. Which ones are paramagnetic? a. Li2 b. C2 c. S2arrow_forwardPredict die molecular structure and bond angles for each molecule or ion in Exercises 88 and 94. a. POCl3, SO42, XeO4, PO43, ClO4 b. NF3, SO32, PO33, ClO3 c.ClO2, SCl2, PCl2 d. Considering your answers to parts a, b, and c. what conclusions can you draw concerning the structures of species containing the same number of atoms and the same number of valence electrons? (O3), sulfur dioxide, and sulfur trioxide.arrow_forward
- Use Lewis structures and VSEPR theory to predict the electron-region and molecular geometries of (a) PSCl3. (b) SOF6. (c) [S2O4]2. (d) [TeF4]2. Note any differences between these geometries.arrow_forwardComplete the following resonance structures for POCl3. a. Would you predict the same molecular structure from each resonance structure? b. What is the hybridization of P in each structure? c. What orbitals can the P atom use to form the bond in structure B? d. Which resonance structure would be favored on the basis of formal charges?arrow_forward
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





