
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 4PP
Practice Problem 13.4
From each set of resonance structures that follow, designate the one that would contribute most to the hybrid and explain your choice:
(a)
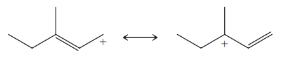
(b)
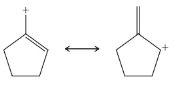
(c)
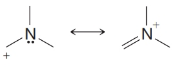
(d)
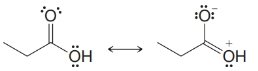
(e)

(f)

Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(f) SO:
Best Lewis Structure
3
e group geometry:_
shape/molecular geometry:,
(g) CF2CF2
Best Lewis Structure
polarity:
e group arrangement:_
shape/molecular geometry:
(h) (NH4)2SO4
Best Lewis Structure
polarity:
e group arrangement:
shape/molecular geometry:
polarity:
Sketch (with angles):
Sketch (with angles):
Sketch (with angles):
1.
Problem Set 3b
Chem 141
For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing
bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the
molecule is polar or non-polar (iv)
(a) SeF4
Best Lewis Structure
e group arrangement:_
shape/molecular geometry:
polarity:
(b) AsOBr3
Best Lewis Structure
e group arrangement:_
shape/molecular geometry:
polarity:
Sketch (with angles):
Sketch (with angles):
(c) SOCI
Best Lewis Structure
2
e group arrangement:
shape/molecular geometry:_
(d) PCls
Best Lewis Structure
polarity:
e group geometry:_
shape/molecular geometry:_
(e) Ba(BrO2):
Best Lewis Structure
polarity:
e group arrangement:
shape/molecular geometry:
polarity:
Sketch (with angles):
Sketch (with angles):
Sketch (with angles):
Chapter 13 Solutions
Organic Chemistry
Ch. 13 - Prob. 1PPCh. 13 - Prob. 2PPCh. 13 - Prob. 3PPCh. 13 - Practice Problem 13.4 From each set of resonance...Ch. 13 - Practice Problem 13.5 The following enol (an...Ch. 13 - Prob. 6PPCh. 13 - Practice Problem 13.7
Two compounds, A and B, have...Ch. 13 - Prob. 8PPCh. 13 - Prob. 9PPCh. 13 - Prob. 10PP
Ch. 13 - Prob. 11PPCh. 13 - Prob. 12PPCh. 13 - Prob. 13PPCh. 13 - Prob. 14PPCh. 13 - Prob. 15PPCh. 13 - Practice Problem 13.16
Diels–Alder reactions also...Ch. 13 - Prob. 17PPCh. 13 - Prob. 18PCh. 13 - What product would you expect from the following...Ch. 13 - Prob. 20PCh. 13 - Prob. 21PCh. 13 - Provide the reagents necessary for each of the...Ch. 13 - Prob. 23PCh. 13 - Prob. 24PCh. 13 - Prob. 25PCh. 13 - When 1-pentene reacts with N-bromosuccinimide...Ch. 13 - Prob. 27PCh. 13 - Prob. 28PCh. 13 - Prob. 29PCh. 13 - Prob. 30PCh. 13 - 13.31 Provide a mechanism that explains formation...Ch. 13 - 13.32 Provide a mechanism that explains formation...Ch. 13 - Treating either 1-chloro-3-methyl-2-butene or...Ch. 13 - Prob. 34PCh. 13 - Prob. 35PCh. 13 - Although both 1-bromobutane and 4-bromo-1-butene...Ch. 13 - Prob. 37PCh. 13 - Prob. 38PCh. 13 - Prob. 39PCh. 13 - Prob. 40PCh. 13 - Prob. 41PCh. 13 - Prob. 42PCh. 13 - Prob. 43PCh. 13 - 13.44 When furan and maleimide undergo a...Ch. 13 - Two controversial hard insecticides are aldrin and...Ch. 13 - Prob. 46PCh. 13 - Prob. 47PCh. 13 - Prob. 48PCh. 13 - Prob. 49PCh. 13 - Prob. 50PCh. 13 - Explain the product distribution below based on...Ch. 13 - Mixing furan (Problem 13.44) with maleic anhydride...Ch. 13 - Prob. 53PCh. 13 - Prob. 54PCh. 13 - Prob. 1LGPCh. 13 - Prob. 2LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
In mechanism, photophosphorylation is most similar to A. substrate-level phosphorylation in glycolysis. B. oxid...
Campbell Biology in Focus (2nd Edition)
The following data were obtained from a disk-diffusion test. Antibiotic Zone of Inhibition A 15 mm B 0 mm c 7 m...
Microbiology: An Introduction
30. Drosophila has a diploid chromosome number of 2n = 8, which includes one pair of sex chromosomes (XX in fem...
Genetic Analysis: An Integrated Approach (3rd Edition)
47. A 250.0-mL buffer solution is 0.250 M in acetic acid and 0.250 M in sodium acetate.
a. What is the initial ...
Chemistry: A Molecular Approach (4th Edition)
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
Describe the various layering arrangements and cell shapes of epithelial tissue.
Principles of Anatomy and Physiology
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY