
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 13, Problem 46P
Interpretation Introduction
Interpretation:
The synthesis of norbornadiene by using cyclopentadiene and acetylene, and cyclopentadiene and vinyl chloride is to be described.
Concept introduction:
Diels-alder is a type of organic reaction in which a substituted
The general reaction of Diels-alder is as follows:
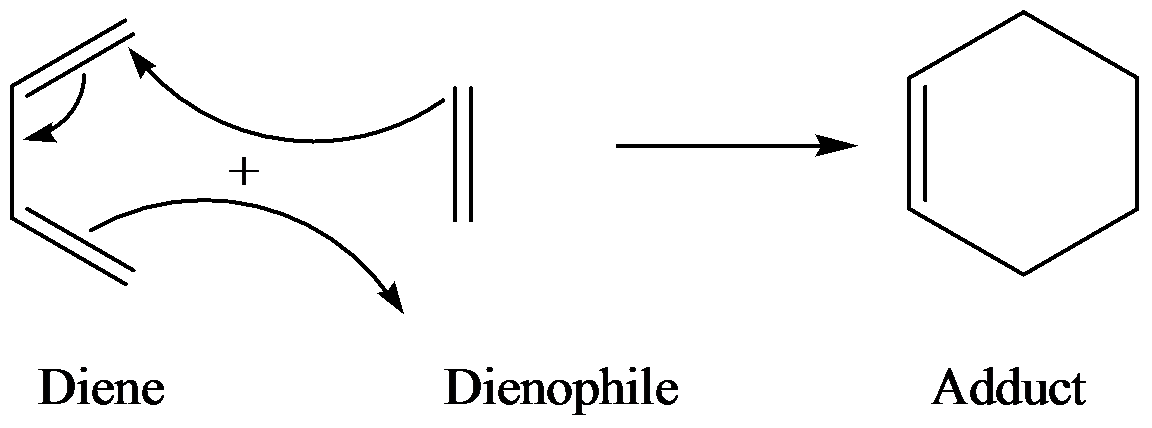
Aldrin is an organic compound that contains chloride groups. It is also known as an organochlorine compound. Aldrin is toxic in nature and is used as insecticides.
Norbornadiene is an organic compound used as dienophile in Diels-alder reaction.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The endiandric acids comprise a group of unsaturated carboxylic acids isolated from a tree that grows in the rainforests of eastern Australia. The methyl esters of endiandric acids D and E have been prepared from polyene Y by a series of two successive electrocyclic reactions: thermal ring closure of the conjugated tetraene followed by ring closure of the resulting conjugated triene. (a) Draw the structures (including stereochemistry) of the methyl esters of endiandric acids D and E. (b) The methyl ester of endiandric acid E undergoes an intramolecular [4 + 2] cycloaddition to form the methyl ester of endiandric acid A. Propose a possible structure for endiandric acid A.
(a) Cyclohexa-1,3-diene can be converted into a tetrasubstituted haloalkane when reacted with bromine in ether. Write a balanced chemical equation for the reaction that occurs and state the expected observation.
(b) Compound A and B are alkenes with the same molecular formula C5H10. Compound A is a branched-chain alkene while compound B is a straight-chain alkene. The reaction between compound A with hydrogen bromide produces major product C which is optically active.
(i) Draw TWO (2) possible structures for compound B.
(ii) Outline the mechanism for the reaction between compound A with hydrogen bromide to form major product C.
(iii) Name the product formed when compound A undergoes bromination reaction.
Write the structure of the major organic product formed in the reaction of 1-pentene with each of the following: (a) Hydrogen chloride (b) Dilute sulfuric acid (c) Diborane in diglyme, followed by basic hydrogen peroxide (d) Bromine in carbon tetrachloride (e) Bromine in water (f) Peroxyacetic acid (g) Ozone (h) Product of part (g) treated with zinc and water (i) Product of part (g) treated with dimethyl sulfide (CH3)2S
Chapter 13 Solutions
Organic Chemistry
Ch. 13 - Prob. 1PPCh. 13 - Prob. 2PPCh. 13 - Prob. 3PPCh. 13 - Practice Problem 13.4 From each set of resonance...Ch. 13 - Practice Problem 13.5 The following enol (an...Ch. 13 - Prob. 6PPCh. 13 - Practice Problem 13.7
Two compounds, A and B, have...Ch. 13 - Prob. 8PPCh. 13 - Prob. 9PPCh. 13 - Prob. 10PP
Ch. 13 - Prob. 11PPCh. 13 - Prob. 12PPCh. 13 - Prob. 13PPCh. 13 - Prob. 14PPCh. 13 - Prob. 15PPCh. 13 - Practice Problem 13.16
Diels–Alder reactions also...Ch. 13 - Prob. 17PPCh. 13 - Prob. 18PCh. 13 - What product would you expect from the following...Ch. 13 - Prob. 20PCh. 13 - Prob. 21PCh. 13 - Provide the reagents necessary for each of the...Ch. 13 - Prob. 23PCh. 13 - Prob. 24PCh. 13 - Prob. 25PCh. 13 - When 1-pentene reacts with N-bromosuccinimide...Ch. 13 - Prob. 27PCh. 13 - Prob. 28PCh. 13 - Prob. 29PCh. 13 - Prob. 30PCh. 13 - 13.31 Provide a mechanism that explains formation...Ch. 13 - 13.32 Provide a mechanism that explains formation...Ch. 13 - Treating either 1-chloro-3-methyl-2-butene or...Ch. 13 - Prob. 34PCh. 13 - Prob. 35PCh. 13 - Although both 1-bromobutane and 4-bromo-1-butene...Ch. 13 - Prob. 37PCh. 13 - Prob. 38PCh. 13 - Prob. 39PCh. 13 - Prob. 40PCh. 13 - Prob. 41PCh. 13 - Prob. 42PCh. 13 - Prob. 43PCh. 13 - 13.44 When furan and maleimide undergo a...Ch. 13 - Two controversial hard insecticides are aldrin and...Ch. 13 - Prob. 46PCh. 13 - Prob. 47PCh. 13 - Prob. 48PCh. 13 - Prob. 49PCh. 13 - Prob. 50PCh. 13 - Explain the product distribution below based on...Ch. 13 - Mixing furan (Problem 13.44) with maleic anhydride...Ch. 13 - Prob. 53PCh. 13 - Prob. 54PCh. 13 - Prob. 1LGPCh. 13 - Prob. 2LGP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (a) Give the products of the phosphoric acid-catalyzed dehydration of 2-methylcyclohex-3- enol. Label the three products with major, minor, and trace amounts. OH H3PO4, heat (b) Describe the rule that is applied in the acid-catalyzed dehydration of 2-methylcyclohex-3- enol. Explain your answer based on the products formed.arrow_forwardDisiamylborane adds only once to alkynes by virtue of its two bulky secondary isoamylgroups. Disiamylborane is prepared by the reaction of BH3 # THF with an alkene.(a) Draw the structural formulas of the reagents and the products in the preparation ofdisiamylborane.(b) Explain why the reaction in part (a) goes only as far as the dialkylborane. Why isSia3B not formed?arrow_forward1. (a) Describe aromaticity, Kekule structure and resonance structure for benzene. (b) Why is benzene more stable than aliphatic alkenes?arrow_forward
- (a) Explain how pyrrole is isoelectronic with the cyclopentadienyl anion.(b) Specifically, what is the difference between the cyclopentadienyl anion and pyrrole?(c) Draw resonance forms to show the charge distribution on the pyrrole structure.arrow_forward11:43 Q1. (a) (c) (d) (b) Two stereoisomers of but-2-ene are formed when 2-bromobutane reacts with ethanolic potassium hydroxide. (i) Explain what is meant by the term stereoisomers. Library Name and outline a mechanism for the reaction of 2-bromo-2-methylpropane with ethanolic potassium hydroxide to form the alkene 2-methylpropene, (CH3)2C=CH₂ Name of mechanism Mechanism (ii) Draw the structures and give the names of the two stereoisomers of but-2-ene. Stereoisomer 1 Name (iii) Name this type of stereoisomerism. Select Name Stereoisomer 2 When 2-bromo-2-methylpropane reacts with aqueous potassium hydroxide, 2-methylpropan-2-ol is formed as shown by the following equation. CH3 H₂C-C-CH3 + KOH Br Page 2 of 14 CH3 H3C-C-CH3 + KBr ОН State the role of the hydroxide ions in this reaction. Write an equation for the reaction that occurs when CH3CH₂CH₂CH₂Br reacts with an excess of ammonia. Name the organic product of this reaction. Equation Name of product 9,284 Photos, 1,166 Videos For You…arrow_forwardDevelop syntheses for the following compounds. As starting materials, you may use cyclopentanol, alcohols containing no more than four carbon atoms, and any common reagents and solvents. (a) trans-cyclopentane-1,2-diol (b) 1-chloro-1-ethylcyclopentanearrow_forward
- (a) The Friedel-Crafts reaction of benzene with 2-chloro-3-methylbutane in the presence of AlCl3 occurs with a carbocation rearrangement. Give mechanistic explanation and the product formed. (b) Predict the product(s) will be formed from the following reactions: (i) Bromination of p-methylbenzoic acid (ii) Sulphonation of m-bromoanisole (iii) Friedel-craft acylation of o-bromonitrobenzenearrow_forwardWrite the formula of reagents used in the following reactions:(i) Bromination of phenol to 2,4,6-tribromophenol(ii) Hydroboration of propene and then oxidation to propanol.(b) Arrange the following compound groups in the increasing order of their property indicated:(i) p-nitrophenol, ethanol, phenol (acidic character)(ii) Propanol, Propane, Propanal (boiling point)arrow_forwardWhich of the following is true for the reactions of alkyl halides? (a) The characteristic reactions of alkyl halides are oxidation and reduction.(b) The characteristic reactions of alkyl halides are elimination and substitutionc. The characteristic reactions of alkyl halides are addition and substitutiond. Characteristic reactions of alkyl halides are addition and elimination.arrow_forward
- Write the structure of the major organic product formed in the reaction of each of the following with hydrogen bromide in the absence of peroxides and in their presence. (a) 1-Pentene (b) 2-Methyl-2-butene (c) 1-Methylcyclohexenearrow_forwardGrignard reagent is a versatile tool in synthetic organic chemistry. Using bromocyclopentane as a starting material, show how a Grignard reagent, X, is synthesized. Reaction of X with water produces compound Y while treatment in carbon dioxide followed by hydrolysis forms compound Z. 3-methyl-2butanone reacts with X and hydrolyses to yield compound AA. Draw the structural formulae of compounds Y, Z and AA and write the chemical equations respectively.arrow_forward(a) Tsomane and Nyiko were given a task of synthesising methylenecyclohexane 2. After a brief discussion with each other, Tsomane proposed Method A to synthesise 2 from cyclohexanone 1 while Nyiko proposed Method B that started from hydroxymethylcyclohexane 3. Each student believed that their proposed method is better than the other. (Scheme below) (1) 1 Ph THF A Ph Ph B H₂SO4 100 °C 3 OH What is the name of the reaction that is followed by reaction Method A?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY