
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 16PP
Practice Problem 13.16
Diels–Alder reactions also take place with triple-bonded (acetylenic) dienophiles. Which diene and which dienophile would you use to prepare the following?
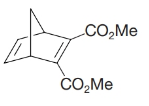
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
When anthracene is added to the reaction of chlorobenzene with concentrated NaOH at 350 °C, an interesting Diels–Alderadduct of formula C20H14 results. The proton NMR spectrum of the product shows a singlet of area 2 around d 3 and abroad singlet of area 12 around d 7. Propose a structure for the product, and explain why one of the aromatic rings ofanthracene reacted as a diene
The diene lactone shown in part (a) has one electron-donating group (¬OR) and one electron-withdrawing group(C“O). This diene lactone is sufficiently electron-rich to serve as the diene in a Diels–Alder reaction.(a) What product would you expect to form when this diene reacts with methyl acetylenecarboxylate, a strong dienophile?
The following triene undergoes an intramolecular Diels-Alder reaction to give a
bicyclic product. Propose a structural formula for the product. Account for the
observation that the Diels-Alder reaction given in this problem takes place under milder
conditions (at lower temperature) than the analogous Diels-Alder reaction
0"C
Diels-Alder adduct
Chapter 13 Solutions
Organic Chemistry
Ch. 13 - Prob. 1PPCh. 13 - Prob. 2PPCh. 13 - Prob. 3PPCh. 13 - Practice Problem 13.4 From each set of resonance...Ch. 13 - Practice Problem 13.5 The following enol (an...Ch. 13 - Prob. 6PPCh. 13 - Practice Problem 13.7
Two compounds, A and B, have...Ch. 13 - Prob. 8PPCh. 13 - Prob. 9PPCh. 13 - Prob. 10PP
Ch. 13 - Prob. 11PPCh. 13 - Prob. 12PPCh. 13 - Prob. 13PPCh. 13 - Prob. 14PPCh. 13 - Prob. 15PPCh. 13 - Practice Problem 13.16
Diels–Alder reactions also...Ch. 13 - Prob. 17PPCh. 13 - Prob. 18PCh. 13 - What product would you expect from the following...Ch. 13 - Prob. 20PCh. 13 - Prob. 21PCh. 13 - Provide the reagents necessary for each of the...Ch. 13 - Prob. 23PCh. 13 - Prob. 24PCh. 13 - Prob. 25PCh. 13 - When 1-pentene reacts with N-bromosuccinimide...Ch. 13 - Prob. 27PCh. 13 - Prob. 28PCh. 13 - Prob. 29PCh. 13 - Prob. 30PCh. 13 - 13.31 Provide a mechanism that explains formation...Ch. 13 - 13.32 Provide a mechanism that explains formation...Ch. 13 - Treating either 1-chloro-3-methyl-2-butene or...Ch. 13 - Prob. 34PCh. 13 - Prob. 35PCh. 13 - Although both 1-bromobutane and 4-bromo-1-butene...Ch. 13 - Prob. 37PCh. 13 - Prob. 38PCh. 13 - Prob. 39PCh. 13 - Prob. 40PCh. 13 - Prob. 41PCh. 13 - Prob. 42PCh. 13 - Prob. 43PCh. 13 - 13.44 When furan and maleimide undergo a...Ch. 13 - Two controversial hard insecticides are aldrin and...Ch. 13 - Prob. 46PCh. 13 - Prob. 47PCh. 13 - Prob. 48PCh. 13 - Prob. 49PCh. 13 - Prob. 50PCh. 13 - Explain the product distribution below based on...Ch. 13 - Mixing furan (Problem 13.44) with maleic anhydride...Ch. 13 - Prob. 53PCh. 13 - Prob. 54PCh. 13 - Prob. 1LGPCh. 13 - Prob. 2LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
Periodic Trends in the Strengths of Acids Choose the stronger acid: (a)H2SorH2Se,(b)H2TeorHI,(c)PH3orNH3. Give ...
Chemistry: The Molecular Nature of Matter
37. Consider the reaction:
Complete the table. Assume that all concentrations are equilibrium concentrat...
Chemistry: Structure and Properties (2nd Edition)
When another atom or group of atoms is substituted for one of the hydrogen atoms in benzene, C2H2 , the boiling...
General Chemistry: Principles and Modern Applications (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The Wittig reaction can be used for the synthesis of conjugated dienes, as, for example, 1-phenyl-1,3-pentadiene. -CH=CHCH CHCH, 1-Phenyl-1,3-pentadiene Propose two sets of reagents that might be combined in a Wittig reaction to give this conjugated diene.arrow_forwardDraw structural formulas for the diene and dienophile that combine in a Diels-Alder reaction to form the product shown. CN Diene + Dienophile CH₂O • Consider E/Z stereochemistry of alkenes. CNarrow_forwardWrite a general rule that can be used to predict the major product of a Diels–Alder reaction between an alkene with an electron-withdrawing substituent and a diene with a substituent that can donate electrons by resonance depending on the location of the substituent on the diene.arrow_forward
- 2) Rank the following dienes by how rapidly they will undergo Diel-Alder reactions with ethylene.arrow_forwardDraw and discuss the mechanism (with arrows to show electron movements) of the Diels-Alder reaction between anthracene and maleic anhydride. Draw the orientation and phases of the reacting p-orbitals showing how they overlap in a “suprafacial” geometry to form productarrow_forward4.35 Formulate the reaction of cyclohexene with (i) Br2 and (ii) meta-chloro- peroxybenzoic acid followed by H30+. Show the reaction intermediates and the final products with correct cis or trans stereochemistry. 4.36 What products would you expect to obtain from reaction of cyclohexa- 1,3-diene with each of the following? (a) 1 mol Br2 in CH2C12 (c) 1 mol DCl (D = deuterium, ²H) (b) 1 mol HCl (d) 2 mol H2 over a Pd catalyst 4.37 Predict the products of the following reactions on hex-1-yne: (a) 1 equiv HBr ? (b) 1 equiv Cl2 ? (c) H2, Lindlar catalystarrow_forward
- The first step in a synthesis of dodecahedrane involves a Diels-Alder reaction between the cyclopentadiene derivative (1) and dimethyl acetylenedicarboxylate (2). Show how these two molecules react to form the dodecahedrane synthetic intermediate (3). + CH,0OCC=CCOOCH, COOCH ČOOCH3 Cyclopentadienyl- cyclopentadiene (1) Dimethyl acetylene- dicarboxylate (2) (3)arrow_forward(a) Draw a Kekulé structure that shows how the reactive positions of anthracene are the ends of a diene, appropriate for a Diels–Alder reaction.(b) The Diels–Alder reaction of anthracene with maleic anhydride is a common organic lab experiment. Predict the product of this Diels–Alder reaction.arrow_forwardFollowing is an example of a type of reaction known as a Diels-Alder reaction 1,3-Pentadiene Ethylene 3-Methylcyclohexene (a racemic mixture) The Diels-Alder reaction between a diene and an alkene is quite remarkable in that it is one of the few ways that chemists have to form two new carbon-carbon bonds in a single reaction. Given what you know about the relative strengths of carbon-carbon sigma and pi bonds, would you predict the Diels-Alder reaction to be exothermic or endothermic? Explain your reasoning.arrow_forward
- The highly reactive triple bond of benzyne is a powerful dienophile. Predict the productof the Diels–Alder reaction of benzyne (from chlorobenzene and NaOH, heated) withcyclopentadiene.arrow_forward14. Please write the major product for each following Diels-Alder reaction. Explain why they are formed by using Frontier Molecular Orbital theory. (a) 1-(diethylamino)-1,3-butadiene + acrylate (b) 2-ethoxy-1,3-butadiene + acrylate Moreover, draw Molecular Orbitals of 1-(diethylamino)-1,3-butadiene, 2-ethoxy-1,3-butadiene & acrylate. In addition, mark energy levels and predict the products by using FMO theory.arrow_forwardAs many as 18 different Diels–Alder products can be obtained by heating a mixture of 1,3-butadiene and 2-methyl-1,3-butadiene. Identify the productsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY