
Concept explainers
Interpretation:
The diene and dienophile for the given products are to be determined.
Concept introduction:
Diels–Alder is a type of organic reaction in which substituted
The general reaction of Diels–Alder is as follows:
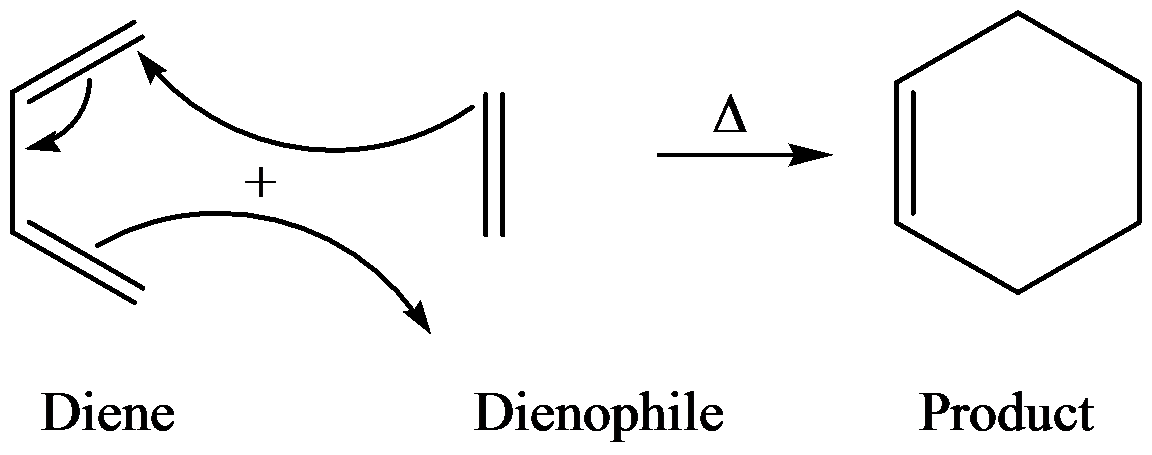
Diels–Alder reaction is between a conjugate diene and a reactant containing double bond (dienophile) to form the product and the product is called adduct.
Diels–Alder reactions are highly stereospecific. The configuration, if dienophile is retained in the product and the reaction, is syn addition.
The dienes react with dienophiles in cis forms rather than trans forms.
Endo and exo refers to the orientation of dienophile and its electron withdrawing group, when it reacts with a diene in Diels–Alder reaction.
The reaction with orientation of electron withdrawing group of dienophiles under the
The reaction with orientation of electron withdrawing group of dienophiles away from the
Endo is favored in the transition state of Diels–Alder reaction because of its lower energy.
If a compound is stable, it has lower energy and if a compound is unstable, it has higher energy.
Trending nowThis is a popular solution!

Chapter 13 Solutions
Organic Chemistry
- (a) CH3CHDOH (b) Show how to make these deuterium-labeled compounds, using CD3MgBr and D2O as your sources of deuterium, and any non-deuterated starting materials you wish. (a) CH3CH(OD)CD3 (d) Ph(CD3)2COD (b) CH3C(OH)(CD3)2 (c) CD3CH2CH2OHarrow_forwardShow how you might synthesize the following compounds, using acetylene and anysuitable alkyl halides as your starting materials. If the compound given cannot besynthesized by this method, explain why.(a) hex-1-yne (b) hex-2-ynearrow_forwardPredict the major products of treating the following compound with hot, concentrated potassium permanganate, followed by acidification with dilute HCl. (a) p-xylenearrow_forward
- (b) Propose how you could synthesize following compounds from suitable alkene. (i) OH (ii) (ii) CH,CH,CHCHCH CH, Br OHarrow_forwardSuggest reagents and reaction conditions for each of the following reactions:arrow_forward4. (A) A medicinal chemist wished to make a series of aromatic molecules bearing a ketone and thioether group with ortho, meta, or para relationships. To achieve this, they propose using nucleophilic aromatic substitution treating the corresponding ortho, meta, and para aryl chlorides with sodium ethanethiolate (NaSEt). Predict which of these reactions will likely work and which will likely fail. Provide a mechanistic explanation why. SET O NasEt EtS glagol Glal ol heat EtS target molecules EtS (B) Would the analogous reactions using EtMgBr instead of NaSEt be more or less likely to work? Explain why or why not. لمسلم EtMgBrarrow_forward
- Starting from bromobenzene and any other reagents and solvents you need, show how you would synthesize the followingcompounds. Any of these products may be used as starting materials in subsequent parts of this problem.(a) 1-phenylpropan-1-olarrow_forwardWhich of the following correctly shows the resonance structures of the resonance stabilized intermediate produced when the shown diene is treated with HBr? (A) (B) (C) (D) HBrarrow_forward3. Outline a synthetic scheme for the preparation of the compounds A from the suggested starting material by the suggested method. Make sure to clearly indicate all reagents needed to perform each step of the synthetic scheme. (a) OH O=S=O Br A (b) H₂N. (c) HO3S. Br Br A A 1 Brarrow_forward
- 2. (a) The reaction below shows bromination of pyrrole. Br2 FeBr3 (i) Give all possible products. (ii) Identify the major product together with your justification.arrow_forwardCompound A is aromatic. It can undergo Friedel-Crafts acylation using acetic anhydride in the presence of Lewis acid tin (IV) chloride. What is the structure of B? Briefly explain the regioselectivity. A (C10H8) (CH3CO)₂0 SnCl4 B (C12H100)arrow_forwardExplain the following observations :(i) The boiling point of ethanol is higher than that of methoxymethane.(ii) Phenol is more acidic than ethanol.(iii) o- and p-nitrophenols are more acidic than phenol.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





