
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 13, Problem 49P
Interpretation Introduction
Interpretation:
The starting material for the given reactions is to be determined.
Concept introduction:
Diels-alder is a type of organic reaction in which a substituted
The general reaction of Diels-alder is as follows:
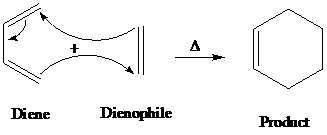
A
The organic reaction in which oxidation of primary alcohol to
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(a)
(b)
(c)
Suggest a synthesis of the following alkene (A) using a Wittig reaction strategy. Draw
the starting material(s), key reagent and a full reaction mechanism including an
explanation of the observed geometry.
Which of the following (B) and (C) will favour the enol form? Briefly explain your
reasoning.
Predict the product(s) and provide a mechanism for each of the following
transformations:
(i)
(ii)
OMe
OMe
Base
OEt
NaOEt
Show how you would synthesize the following compounds, starting with benzene or toluene and any necessary acyclic reagents. Assume para is the major product (and separable from ortho) in ortho, para mixtures. (a) 3-nitro-4-bromobenzoic acid (b) 3-nitro-5-bromobenzoic acid (c) 4-butylphenol (d) 2-(4-methylphenyl)butan-2-ol
Write equations showing how to prepare each of the following from benzene or toluene and any necessary organic or inorganic reagents. If an ortho, para mixture is formed in any step of your synthesis, assume that you can separate the two isomers. (a) Isopropylbenzene (b) p-Isopropylbenzenesulfonic acid (c) 2-Bromo-2-phenylpropane (d) 4-tert-Butyl-2-nitrotoluene (e) m-Chloroacetophenone (f) p-Chloroacetophenone (g) 3-Bromo-4-methylacetophenone (h) 2-Bromo-4-ethyltoluene (i) 3-Bromo-5-nitrobenzoic acid (j) 2-Bromo-4-nitrobenzoic acid (k) 1-Phenyloctane (l) 1-Phenyl-1-octene (m) 1-Phenyl-1-octyne (n) 1,4-Di-tert-butyl-1,4-cyclohexadiene
Chapter 13 Solutions
Organic Chemistry
Ch. 13 - Prob. 1PPCh. 13 - Prob. 2PPCh. 13 - Prob. 3PPCh. 13 - Practice Problem 13.4 From each set of resonance...Ch. 13 - Practice Problem 13.5 The following enol (an...Ch. 13 - Prob. 6PPCh. 13 - Practice Problem 13.7
Two compounds, A and B, have...Ch. 13 - Prob. 8PPCh. 13 - Prob. 9PPCh. 13 - Prob. 10PP
Ch. 13 - Prob. 11PPCh. 13 - Prob. 12PPCh. 13 - Prob. 13PPCh. 13 - Prob. 14PPCh. 13 - Prob. 15PPCh. 13 - Practice Problem 13.16
Diels–Alder reactions also...Ch. 13 - Prob. 17PPCh. 13 - Prob. 18PCh. 13 - What product would you expect from the following...Ch. 13 - Prob. 20PCh. 13 - Prob. 21PCh. 13 - Provide the reagents necessary for each of the...Ch. 13 - Prob. 23PCh. 13 - Prob. 24PCh. 13 - Prob. 25PCh. 13 - When 1-pentene reacts with N-bromosuccinimide...Ch. 13 - Prob. 27PCh. 13 - Prob. 28PCh. 13 - Prob. 29PCh. 13 - Prob. 30PCh. 13 - 13.31 Provide a mechanism that explains formation...Ch. 13 - 13.32 Provide a mechanism that explains formation...Ch. 13 - Treating either 1-chloro-3-methyl-2-butene or...Ch. 13 - Prob. 34PCh. 13 - Prob. 35PCh. 13 - Although both 1-bromobutane and 4-bromo-1-butene...Ch. 13 - Prob. 37PCh. 13 - Prob. 38PCh. 13 - Prob. 39PCh. 13 - Prob. 40PCh. 13 - Prob. 41PCh. 13 - Prob. 42PCh. 13 - Prob. 43PCh. 13 - 13.44 When furan and maleimide undergo a...Ch. 13 - Two controversial hard insecticides are aldrin and...Ch. 13 - Prob. 46PCh. 13 - Prob. 47PCh. 13 - Prob. 48PCh. 13 - Prob. 49PCh. 13 - Prob. 50PCh. 13 - Explain the product distribution below based on...Ch. 13 - Mixing furan (Problem 13.44) with maleic anhydride...Ch. 13 - Prob. 53PCh. 13 - Prob. 54PCh. 13 - Prob. 1LGPCh. 13 - Prob. 2LGP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nucleophilic aromatic substitution provides one of the common methods for making phenols. ) Show how you would synthesize the following phenols, using benzene or toluene as your aromatic starting material, and explain why mixtures of products would be obtained in some cases. (a) m-cresol (b) p-n-butylphenolarrow_forwardApply retrosynthetic analysis to guide the preparation of each of the following compounds from the indicated starting material, then write out the synthesis showing the necessary reagents. (a) 1-Propanol from 2-propanol (b) 1,2-Dibromopropane from 2-bromopropane (c) 1-Bromo-2-propanol from 2-propanol (d) 1-Bromo-2-methyl-2-propanol from tert-butyl bromide (e) 1,2-Epoxypropane from 2-propanol (f) tert-Butyl alcohol from isobutyl alcohol (g) tert-Butyl iodide from isobutyl iodide (h) trans-2-Chlorocyclohexanol from cyclohexyl chloridearrow_forwardWhich reactions will produce the desired product in good yield? You may assume thataluminum chloride is added as a catalyst in each case. For the reactions that will not givea good yield of the desired product, predict the major products.Reagents Desired Product(a) benzene + n-butyl bromide n-butylbenzene(b) ethylbenzene + tert-butyl chloride p-ethyl-tert-butylbenzenearrow_forward
- Ethoxide is used as the base in the condensation of ethyl acetate to avoid some unwantedside reactions. Show what side reactions would occur if the following bases were used.(a) sodium methoxide (b) sodium hydroxidearrow_forwardPredict the major product or the necessary reagent or reactant to complete each of the following reactions. In the box before each reaction, indicate the mechanism followed by the reaction. (Free radical (FR), SN2, SN1). (b) (a) Cl₂ CH₂CH₂OH (a) 1 hv 2 (b) H Br H₂O Na+ SCH3 (c) (d) CI "CH3arrow_forward(a) Although phenoxide ion has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid than phenol. Give two reasons.(b) How will you bring about the following converstions?(i) Propanone to propane (ii) Benzoyl chloride to benzaldehyde(iii) Ethanal to but-2-enalarrow_forward
- (a) CH3CHDOH (b) Show how to make these deuterium-labeled compounds, using CD3MgBr and D2O as your sources of deuterium, and any non-deuterated starting materials you wish. (a) CH3CH(OD)CD3 (d) Ph(CD3)2COD (b) CH3C(OH)(CD3)2 (c) CD3CH2CH2OHarrow_forwardGuiding your reasoning by retrosynthetic analysis, show how you could prepare each of the following compounds from the given starting material and any necessary organic or inorganic reagents. All require more than one synthetic step. (a) Cyclopentyl iodide from cyclopentane (b) 1-Bromo-2-methylpropane from 2-bromo-2-methylpropane (c) meso-2,3-Dibromobutane from 2-butyne (d) 1-Heptene from 1-bromopentane (e) cis-2-Hexene from 1,2-dibromopentane (f) Butyl methyl ether (CH3CH2CH2CH2OCH3) from 1-butenearrow_forwardNucleophilic aromatic substitution provides one of the common methods for making phenols. Show how you would synthesize the following phenols, using benzene or toluene as your aromatic starting material, and explain why mixtures of products would be obtained in some cases.(a) p-nitrophenol (b) 2,4,6-tribromophenol (c) p-chlorophenolarrow_forward
- Show how you would prepare cyclopentene from each compound.(a) cyclopentanol(b) cyclopentyl bromidearrow_forward2B Suggest a short synthetic route for the preparation of compound D from compound C OH Br COOH C D Note: Apart from compound C, you can also use organic reagents with up to 1 C atom. The number of arrows in the figure above does not necessarily correspond to the number of steps.arrow_forward(c) Answer each of the questions below that relate to acetophenone: Xo (i) (ii) (iii) Draw the structure of the enol form of acetophenone. Give a stepwise mechanism for the conversion of acetophenone into its enol form. Show how each of the three compounds A, B and C below can be prepared from acetophenone. Explain clearly what reactants/reagents would be required in each case. odocor A B Br Carrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY