
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 4PP
PRACTICE PROBLEM 12.4
Predict the products of the following acid–base reactions. Using
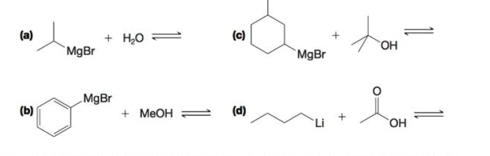
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
19.58 For each of the following questions, please provide a route that could reasonably be expected to convert the starting material into
the product. In each case, more than one reaction is required, and reactions you have learned in previous chapters may be needed to solve
the problem.
-8 8
(b)
Jos 1-3
(d)
NH₂
OEt
OEt
OEt
Eto
OH
LOH
5-
OH
H₂N
OH
"NH₂
O
HO
CH30 Na
A
+
B
O,N
pK =7
(a) Provide structures for A and B (including nonbonding electron pairs and formal
charges where necessary), where A is the conjugate acid and B is the conjugate base
of the reaction.
Answer:
(b) Provide and arrow pushing mechanism to show how A and B are formed.
Answer:
(c) Will the reaction proceed from left to right (i.e. are A and B favoured at equilibrium)?
Explain your answer.
Answer:
(d) Draw all important resonance contributors of the conjugate base B. Rank the
contributors from most stable to least stable (if contributors are equally stable indicate
this with an equals sign)
Rank the following compounds in their correct order of acidity. 1=Most acidic and 4=least acidic.
Chapter 12 Solutions
Organic Chemistry
Ch. 12 - Prob. 1PPCh. 12 - Prob. 2PPCh. 12 - Prob. 3PPCh. 12 - PRACTICE PROBLEM 12.4 Predict the products of the...Ch. 12 - Prob. 5PPCh. 12 - Prob. 6PPCh. 12 - Practice Problem 12.7
Provide retrosynthetic...Ch. 12 - Prob. 8PPCh. 12 - What products would you expect from the reaction...Ch. 12 - What products would you expect from the reaction...
Ch. 12 - What product (or products) would be formed from...Ch. 12 - Prob. 12PCh. 12 - 12.13 Write reaction conditions and the product...Ch. 12 - Prob. 14PCh. 12 - Predict the organic product from each of the...Ch. 12 - Predict the organic product from each of the...Ch. 12 - Predict the organic product from each of the...Ch. 12 - Predict the major organic product from each of the...Ch. 12 - Synthesize each of the following compounds from...Ch. 12 - Prob. 20PCh. 12 - 21. Write a mechanism for the following reaction....Ch. 12 - Prob. 22PCh. 12 - 23. What organic products A-H would you expect...Ch. 12 - Prob. 24PCh. 12 - Show how 1-pentanol could be transformed into each...Ch. 12 - Provide the reagents needed to accomplish...Ch. 12 - Prob. 27PCh. 12 - For each of the following alcohols, write a...Ch. 12 - Prob. 29PCh. 12 - Prob. 30PCh. 12 - Prob. 31PCh. 12 - Prob. 32PCh. 12 - Predict the major organic product from each of the...Ch. 12 - 34. Synthesize the following compound using...Ch. 12 - Prob. 35PCh. 12 - Prob. 36PCh. 12 - 37. Explain how and IR spectroscopy could be used...Ch. 12 - 38. An unknown X shows a broad absorption band in...Ch. 12 - Prob. 39PCh. 12 - The problem below is directed toward devising a...
Additional Science Textbook Solutions
Find more solutions based on key concepts
1.3 Obtain a bottle of multivitamins and read the list of ingredients. What are four chemicals from the list?
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
83. Which solid has the highest melting point? Why?
a. Ar(s)
b.
c. LiCl(s)
d.
Introductory Chemistry (6th Edition)
Use electronegativities to predict the direction of the dipole moments of the following bonds. a. CCl b. CO c. ...
Organic Chemistry (9th Edition)
16.43 The following pictures represent solutions at various stages in thetitration of a weak diprotic acid with...
Chemistry (7th Edition)
Determine the number of protons, neutrons, and electrons in the following atoms: a. a hydrogen atom that has a ...
General, Organic, and Biological Chemistry (3rd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Arrange the following compounds in the increasing order of their acid strength: p-cresol, p-nitrophenol, phenolarrow_forwardThe table shows the base ionisation constant, Kb, for several selected compounds. (a) Compound C6H5NH2 Kb 3.8x10-10 1.7x10-6 N2H4 NH3 1.8x10-5 NH2OH 1.1x10-8 i. Arrange the compounds in order of increasing strength of base. ii. Give the structure of conjugate acid for each compound and arrange them in order of increasing strength of acid. (b) The percentage ionisation of 0.010 M NH3 solution was 4.2 % ionisation. Calculate Kb. 1.8x105arrow_forwardH3C. OH HO Na + pK = 9 (a) Provide structures for A and B (including nonbonding electron pairs and formal charges where necessary), where A is the conjugate acid and B is the conjugate base of the reaction. Answer: (b) Provide and arrow pushing mechanism to show how A and B are formed. Answer: (c) Will the reaction proceed from left to right (i.e. are A and B favoured at equilibrium)? Explain your answer. Answer: (d) Draw all important resonance contributors of the conjugate base B.arrow_forward
- Using pKa Values to Determine Relative Acidity and Basicity Rank the following compounds in order of increasing acidity, and then rank their conjugate bases in order of increasing basicity.arrow_forwardDraw the product of the following Lewis acid-base reaction. Discuss whether the product will retain its monomeric form or if it will dimerise and why. (c) Ph Toluene AICI CHO Pharrow_forwardIdentify the most and the least acidic compound in each of the following sets. Leave the remaining answer in each set blank. a) 2,4-dinitrobenzoic acid: v p-nitrobenzoic acid: p-bromobenzoic acid: b) benzoic acid: v formic acid: v propanoic acid: c) cyclohexanol: v phenol: v benzoic acid:arrow_forward
- 12.6 (opq) Predict the major product and give the name of the reactionarrow_forward(a) Tsomane and Nyiko were given a task of synthesising methylenecyclohexane 2. After a brief discussion with each other, Tsomane proposed Method A to synthesise 2 from cyclohexanone 1 while Nyiko proposed Method B that started from hydroxymethylcyclohexane 3. Each student believed that their proposed method is better than the other. (Scheme below) (1) 1 Ph THF A Ph Ph B H₂SO4 100 °C 3 OH What is the name of the reaction that is followed by reaction Method A?arrow_forwardState whether the following reactions are favorable or unfavorable. Explain the reason behind your answer.arrow_forward
- (a) Tsomane and Nyiko were given a task of synthesising methylenecyclohexane 2. After a brief discussion with each other, Tsomane proposed Method A to synthesise 2 from cyclohexanone 1 while Nyiko proposed Method B that started from hydroxymethylcyclohexane 3. Each student believed that their proposed method is better than the other. (Scheme below) (1) Ph Ph 836 Ph THF A 1 B H₂SO4 100 °C 3 OH Which of the two methods (A or B) is more likely give high yield of methylenecyclohexane 2? Briefly explain.arrow_forwardPredict the reagents for reactions A and B.arrow_forward(a) Tsomane and Nyiko were given a task of synthesising methylenecyclohexane 2. After a brief discussion with each other, Tsomane proposed Method A to synthesise 2 from cyclohexanone 1 while Nyiko proposed Method B that started from hydroxymethylcyclohexane 3. Each student believed that their proposed method is better than the other. (Scheme below) Ph THF A Ph Ph B H₂SO4 100 °C 3 OH (iii) In analysing both these methods, are there other possible alkene products other than methylenecyclohexane 2? Use mechanistic details to support your answer.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY