
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 10P
What products would you expect from the reaction of propyllithium
(a)
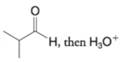
(b)
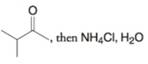
(c)
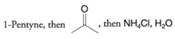
(d) Ethanol
(e)
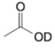
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
2. How would you prepare the following substances from 2-cyclohexenone? More than one
step may be needed.
(a) Cyclohexene
(b) 3-Phynylcyclohexanone
(c) 3-Oxocyclohenanecarboxylic acid
(d) Methyleyclohexane
4 Predict the products of reactions of ethers and epoxides, including the following:(a) Cleavage and autoxidation of ethers(b) Acid- and base-promoted opening of epoxides(c) Reactions of epoxides with organometallic reagents(d) Cleavage of silyl ethers
In an aqueous solution containing sodium bicarbonate, aniline reacts quickly withbromine to give 2,4,6-tribromoaniline. Nitration of aniline requires very strong conditions,however, and the yields (mostly m-nitroaniline) are poor.(a) What conditions are used for nitration, and what form of aniline is present under theseconditions?
Chapter 12 Solutions
Organic Chemistry
Ch. 12 - Prob. 1PPCh. 12 - Prob. 2PPCh. 12 - Prob. 3PPCh. 12 - PRACTICE PROBLEM 12.4 Predict the products of the...Ch. 12 - Prob. 5PPCh. 12 - Prob. 6PPCh. 12 - Practice Problem 12.7
Provide retrosynthetic...Ch. 12 - Prob. 8PPCh. 12 - What products would you expect from the reaction...Ch. 12 - What products would you expect from the reaction...
Ch. 12 - What product (or products) would be formed from...Ch. 12 - Prob. 12PCh. 12 - 12.13 Write reaction conditions and the product...Ch. 12 - Prob. 14PCh. 12 - Predict the organic product from each of the...Ch. 12 - Predict the organic product from each of the...Ch. 12 - Predict the organic product from each of the...Ch. 12 - Predict the major organic product from each of the...Ch. 12 - Synthesize each of the following compounds from...Ch. 12 - Prob. 20PCh. 12 - 21. Write a mechanism for the following reaction....Ch. 12 - Prob. 22PCh. 12 - 23. What organic products A-H would you expect...Ch. 12 - Prob. 24PCh. 12 - Show how 1-pentanol could be transformed into each...Ch. 12 - Provide the reagents needed to accomplish...Ch. 12 - Prob. 27PCh. 12 - For each of the following alcohols, write a...Ch. 12 - Prob. 29PCh. 12 - Prob. 30PCh. 12 - Prob. 31PCh. 12 - Prob. 32PCh. 12 - Predict the major organic product from each of the...Ch. 12 - 34. Synthesize the following compound using...Ch. 12 - Prob. 35PCh. 12 - Prob. 36PCh. 12 - 37. Explain how and IR spectroscopy could be used...Ch. 12 - 38. An unknown X shows a broad absorption band in...Ch. 12 - Prob. 39PCh. 12 - The problem below is directed toward devising a...
Additional Science Textbook Solutions
Find more solutions based on key concepts
The following reaction has a value of G = 2.1kJ/mol(0.50kcaI/mol). CH3Br + H2S CH3 SH + HBr a. Calculate Keq a...
Organic Chemistry (9th Edition)
Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2
Chemistry
Conceptual PRACTICE 18.18 Consider the following galvanic cell:
(a) What is the change in the cell voltage on ...
Chemistry (7th Edition)
For Practice 1.1
Is each change physical or chemical? Which kind of property (chemical or physical) is demonst...
Principles of Chemistry: A Molecular Approach (3rd Edition)
23. Give the symbol and name for (a) an isotope with a mass number of 37 and an atomic number of 17 and (b) an ...
Chemistry For Changing Times (14th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 22). What is the major product of the following reactions? (A) (B) Br 1) NaCN 2) CH3MgBr 3) H30*/H₂O (C) LOH (D) CNarrow_forwardProvide the major product for the following reaction? (1) BH3, ether (2) H2O2, OHarrow_forwardWHAT PRODUCTS WOULD YOU OBTAIN FROM REACTION OF BUTAN-1-OL WITH THE FOLLOWING REAGENTS? (а) PBr3 (b) CrOз, H30 (c) Na (d) A periodinanearrow_forward
- Predict the products from reaction of 2-hexyne with the following reagents:(a) 2 equiv Br2(b) 1 equiv HBr(c) Excess HBr(d) Li in NH3(e) H2O, H2SO4, HgSO4arrow_forwardPredict the products formed when cyclohexanecarbaldehyde reacts with the following reagents.(a) PhMgBr, then H3O+ (b) Tollens reagent (c) semicarbazide and weak acid(d) excess ethanol and acid (e) propane-1,3-diol, H+ (f) zinc amalgam and dilute hydrochloric acidarrow_forward9. Plan syntheses of the following compounds. You may use the given starting material and any compound containing three or fewer carbons. (a) (b) (c) (d) by Br or Br H Br H OH = OHarrow_forward
- Predict the major products formed when benzoyl chloride (PhCOCl) reacts with the following reagents.(a) ethanol (b) sodium acetate (c) anilinearrow_forward1. At what position and on what ring would you expect the following substances to undergo electrophilic substitution? (b) CH3 Br lel CH3 2. Rank the compounds in each group according to their reactivity toward electrophilic substitution. (a) Chlorobenzene, o-dichlorobenzene, benzene (b) p-Bromonitrobenzene, nitrobenzene, phenol (c) Fluorobenzene, benzaldehyde, 0-xylene (d) Benzonitrile, p-methylbenzonitrile, p-methoxybenzonitrilearrow_forwardAldehydes and ketones react with thiols to yield thioacetals just as they react with alcohols to yield acetals. Predict the product of the following reaction, and propose a mechanism:arrow_forward
- Compound H (C8H6O3) gives a precipitate when treated with hydroxylamine in aqueous ethanol and a silver mirror when treated with Tollens solution. Following is its 1H-NMR spectrum. Deduce the structure of compound H.arrow_forwardNaH HO HO PBR3 A (a) Name the functional groups in compounds A and B, including, if relevant, whether they are primary (1°), secondary (2°) or tertiary (3'). (b) Propose a reagent that could be used to convert A into B. (c) Draw the structure of the intermediate C formed by reaction of compound A with NaH. (d) Propose the structure of the organic compound D formed by reaction with PB.3. (e) Could HBr be used for the conversion of A into D? Explain very briefly.arrow_forward(S)-2-butanol reacts with potassium dichromate (K2CrO4) in aqueous sulfuric acid to give A(C,HgO). Treatment of A with ethylmagnesium bromide in anhydrous ether gives B(C,H140). Draw the structure of B.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License